Assembly area
FOREWORD
By Lorraine Nolan
Chair of EMA Management Board
INTRODUCTION
By Emer Cooke
EMA Executive Director
CHAPTER 1
KEY ACHIEVEMENTS IN 2023
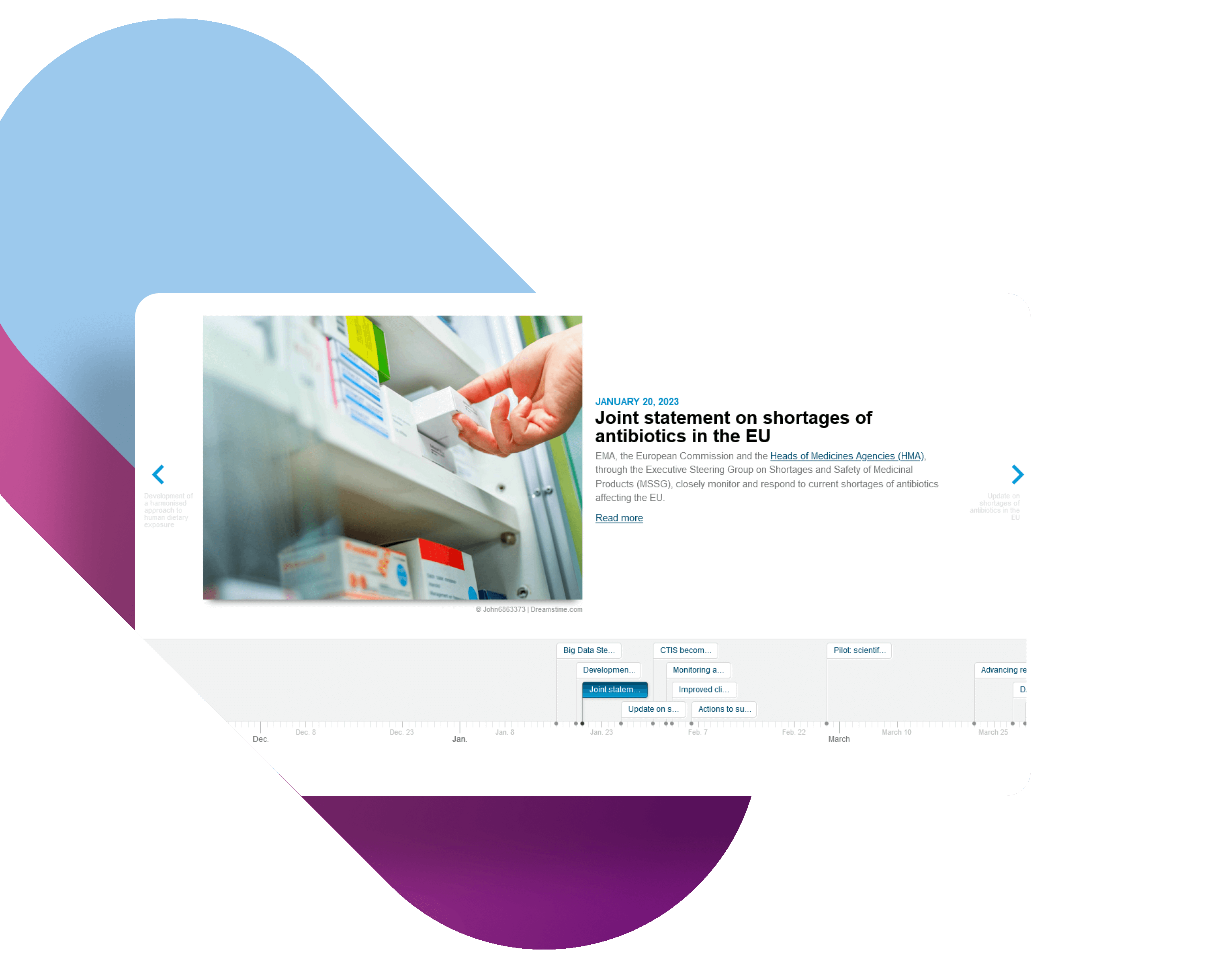
The first chapter focuses on EMA’s strategic priorities and contributions to public and animal health in Europe. Click on the links below to learn more about the most important highlights in the evaluation and monitoring of human and veterinary medicines and major achievements in 2023.
EMA by the numbers
Key figures on EMA’s recommendations for the authorisation of new medicines in 2023:
0
positive opinions for human medicines
0
human medicines with a new active substance
0
negative opinions for human medicines
0
positive opinions for veterinary medicines
0
veterinary medicines with a new active substance
0
negative opinions for veterinary medicines
CHAPTER 2
KEY FIGURES IN 2023
The second chapter presents a selection of key figures and trends illustrating EMA’s activities in the regulation of medicines in the EU. Click on the links below for core statistics.