.png)
European medicines regulatory network
The European medicines regulatory network is the cornerstone of EMA's work and success. EMA operates at the heart of this network, coordinating and supporting interactions between over 50 national competent authorities in the EU and the EEA for both human and veterinary medicines.
The network gives EMA access to a pool of over 4,000 experts, who provide the best available scientific expertise for the regulation of medicines in the EU by participating in EMA’s scientific committees, working parties and other groups as well as members of the assessment teams carrying out the evaluation of medicines.
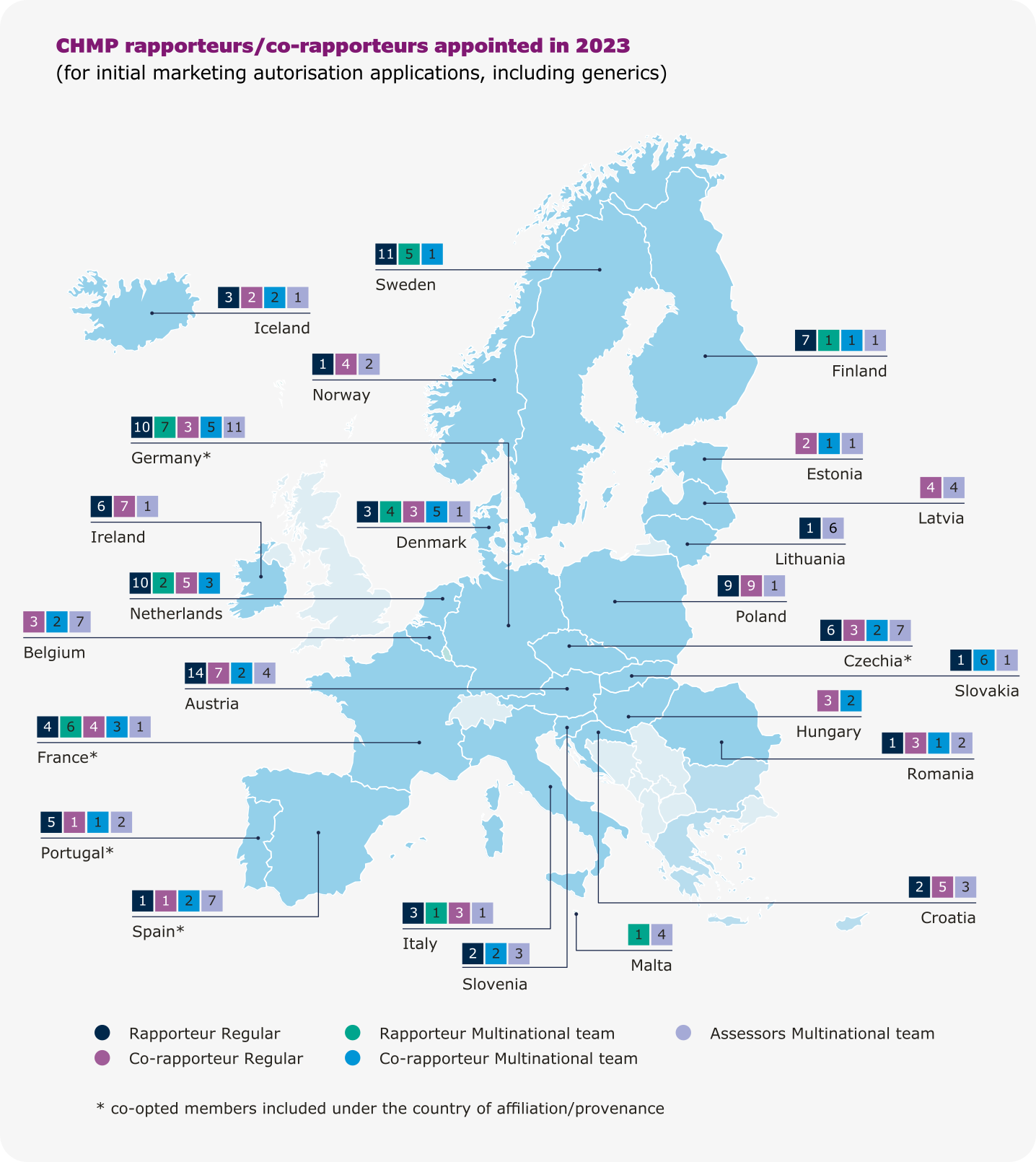
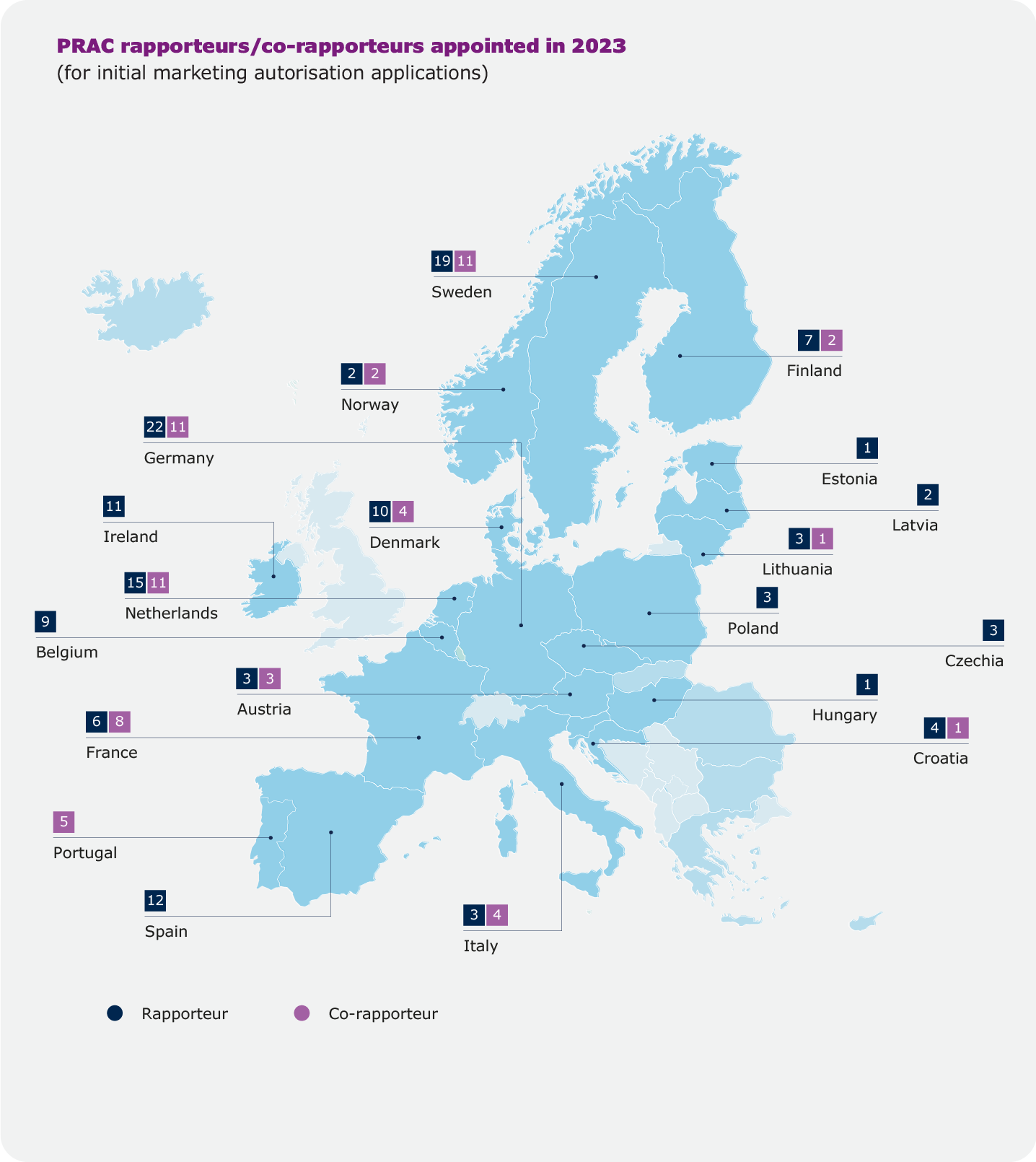
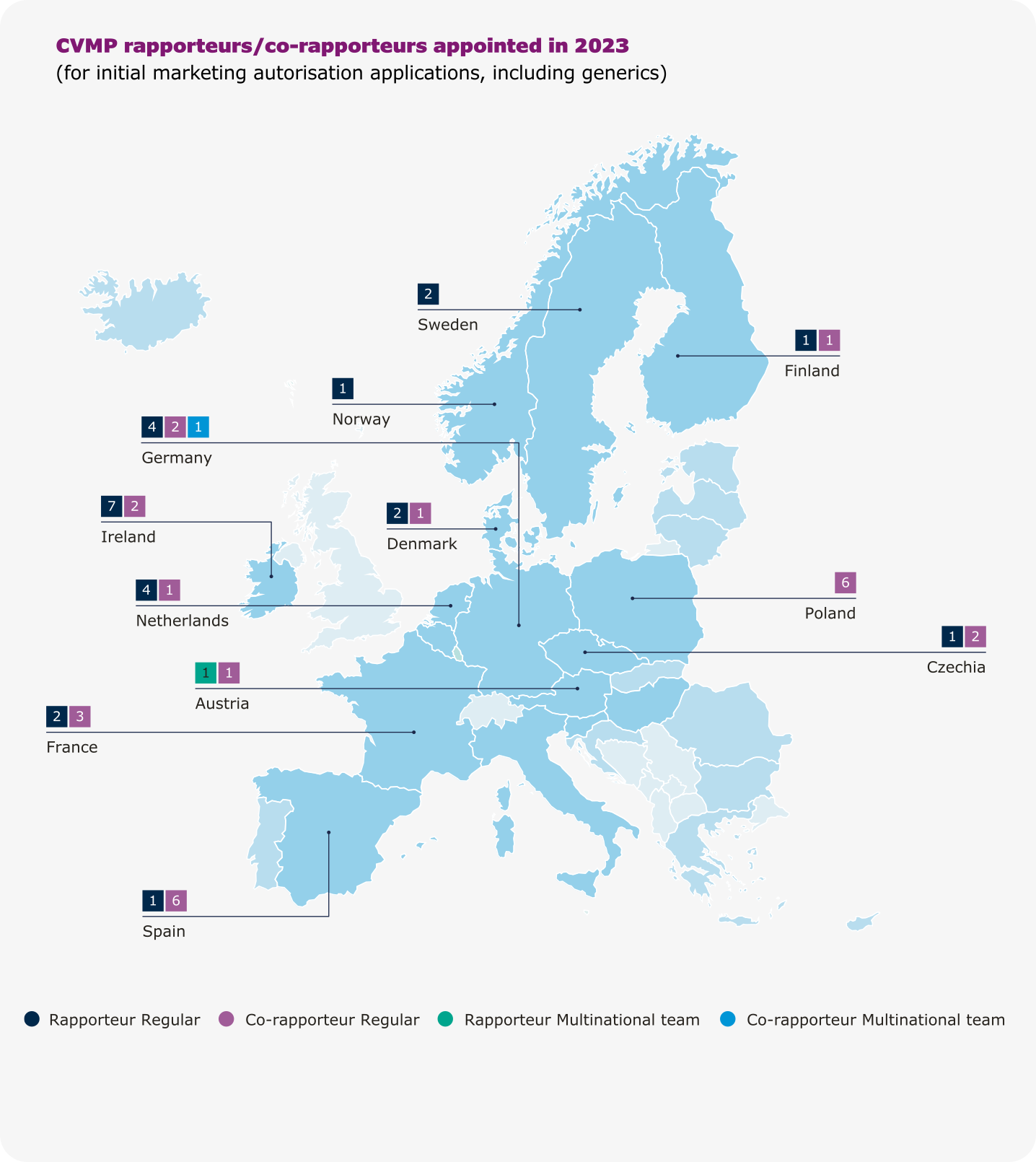
Rapporteurships and co-rapporteurships
The assessment of a medicine by EMA’s scientific committees is carried out by a rapporteur and a co-rapporteur, who prepare the assessment reports and lead discussions in the committees. The appointment is made on the basis of the best possible expertise for the particular product. Rapporteurs work through assessment procedures and take the lead in evaluating any new information on the medicine that may become available.