EMA continues to provide updates to stakeholders on further changes to the Eudravigilance system.
Guidance on using ISO standard format for individual case safety reports
As of 30 June 2022, EudraVigilance users need to report individual cases of suspected side effects using the ISO ICSR/ICH E2B(R3)format and related ISO standard terminology for pharmaceutical form and route of administration.
Revised guidance on the technical requirements and process for transmitting individual case safety reports (ICSRs) is available below. This helps pharmaceutical companies, sponsors of clinical trials and medicines regulatory authorities in EU Member States prepare to use the new ICSR standard.
The revised guidance also includes updates on:
- the EudraVigilance registration process;
- the EudraVigilance business rules;
- data protection (i.e. legal references to the General Data Protection Regulation (GDPR) and the EU Data Protection Regulation (DPR);
- changes to reporting requirements for clinical trials that will apply after the go-live of the Clinical Trials Information System (CTIS).
The new business rules are available in the EudraVigilance test environment (XCOMP). They enable stakeholders to test changes to their own systems.
For more information, see:
| Feature added in 2017 | Benefit |
|---|---|
|
|
|
|
|
|
|
|
|
|
| Stakeholder | Requirement |
|---|---|
| EMA |
|
| Marketing authorisation holders |
|
| All stakeholders within the European Union (EU) medicines regulatory network |
|
The main technical change to the EudraVigilance system was the implementation of the new ISO ICSR format for the submission and exchange of ICSRs in line with the applicable ICH E2B(R3) and EU ICSR Implementation Guides.
To incorporate the new format, EMA implemented several other changes in EudraVigilance. These include:
- adapting the EudraVigilance Gateway, the secure electronic communication tool to exchange ICSRs, to accept ICH E2B(R2) as well as (R3) files;
- adapting the EudraVigilance database management system (EDBMS) to support the storage and processing of reports submitted with the new ICH ICSR format
- migrating all existing ICH E2B(R2) ICSR data to the new ICH ICSR format;
- redesign of EVWEB, the web application for the electronic reporting and management of ICSRs in the ISO ICSR format, using a new technology;
- introducing new functionalities in the EudraVigilance data analysis system (EVDAS), including the adrreports.eu portal, to support analysis of data and support signal management activities.
In addition, EMA added the following two new functionalities to the system:
- EudraVigilance rerouting functionality, which defines the rules for the forwarding of ICSRs to the national competent authority (NCA) where the adverse reaction occurred (rules can be set by NCAs according to their needs and preferences);
- ICSR download functionality to enable marketing authorisation holders to download ICSRs concerning their products or ICSRs reported with a substance for which they hold a marketing authorisation in the EEA.
The extended EudraVigilance medicinal product dictionary is no longer an integral part of EudraVigilance version 8 but continues to be available as an independent system component.
The implementation of the new EudraVigilance system changed the following business processes:
- Marketing authorisation holders no longer provide ICSRs to national competent authorities and submit these to EudraVigilance only.
- Following the switch to simplified reporting, EMA submits ICSRs through EudraVigilance to the WHO Uppsala Monitoring Centre, rather than national competent authorities doing this;
- Marketing authorisation holders have a legal obligation to monitor the data available in EudraVigilance and inform EMA or national competent authorities of any safety signals identified. EMA granted marketing authorisation holders access to EVDAS to use signal detection, analytical and reporting functions to the extent necessary to fulfil their obligation.
For more in-depth analysis of the changes to each stakeholder group, see:
All impacted organisations should have prepared for the changes in reporting, downloading and analysis of data before the new EudraVigilance system was moved into production.
National competent authorities should have planned communication activities at national level to ensure that marketing authorisation holders in their territory were aware of the changes and had systems in place to comply with their obligation to report directly to EudraVigilance, prior to 22 November 2017.
Guidance
A compilation of guidance is available below on the enhanced EudraVigilance system launched in 2017.
For reference, this includes obsolete guidance on the use of the ICH E2B(R2) format for reporting individual cases of suspected side effects, which was possible until 30 June 2022.
As of 30 June 2022, EudraVigilance users need to report individual cases of suspected side effects using the ISO ICSR/ICH E2B(R3)format. For more information, see Guidance on using ISO standard format for individual case safety reports.
ICH provides the basis for the implementation of the ISO ICSR standard. Relevant documents are downloadable from the ICH Electronic Standards for the Transfer of Regulatory Information (ESTRI) website. They are also listed below.
To complement the ICH E2B(R3) implementation guides, EMA has developed a specific EU ICSR implementation guide and supporting documents, including user guides and training material. These documents provide additional information on specific EU regional requirements not provided for in the ICH documentation.
ICH implementation guidance
| Documentation | Description |
|---|---|
| ICH Implementation guide package | Set of documents including the ICH ICSR implementation guide, backwards and forwards compatibility recommendations and element mapping. |
| ICH E2B(R3) Questions and answers | Relevant for technical E2B questions. |
EU implementation guidance
| Documentation | Description |
|---|---|
| European Union individual case safety report (ICSR) implementation guide | This guidance describes the EU-specific requirements to generate a valid ICSR safety and acknowledgment message in the international format EN ISO ICSR 27953-2:2011 in accordance with ICH E2B(R3) guidance. |
| EU Individual Case Safety Report (ICSR) implementation guide business rules spreadsheets | These spreadsheets include all the ICH E2B(R3) and EU specific business rules in a format to help system developers. |
| European Union backwards forwards conversion element mapping spreadsheet | This document describes the relationship between EU specific data elements in E2B(R3) and E2B(R2). This document is an addition to the ICH backwards-forwards conversion rules. It covers additional EU-specific rules for the conversion back and forth between E2B(R2) and E2B(R3). |
| European Union BFC conversion v.2.8 | The ICH backwards-forwards conversion tool updated to include additional EU-specific data fields. |
| European Union E2B (R3) code lists | The list of codes for EU-specific data fields. |
| EU R2 Dosages to EDQM mapping | This list provides a mapping of the previously published EU R2 dosage forms |
| UCUM units for E2B (R3) version 1.2 | The list of valid UCUM units that can be used in the EU for E2B (R3). |
| European Union reference instances | ICH reference instances amended to include EU-specific data fields. |
| European Union example instances - E2B(R3) testing files | Additional example instances to be used for testing E2B(R3) transmissions with the enhanced EudraVigilance system. |
Revised EudraVigilance access policy
| Documentation | Description |
|---|---|
| European Medicines Agency policy on access to EudraVigilance data for medicinal products for human use - Revision 3 (current) | The revised EudraVigilance access policy, which governs the level of access different stakeholder groups have to adverse drug reactions reports. The revised version came into force on 22 November 2017. |
| Overview of comments received on 'Draft revision of EudraVigilance access policy for medicines for human use' | The comments received on the draft revision of the EudraVigilance access policy during its public consultation between 4 August and 15 September 2014. |
Good pharmacovigilance practice (GVP) guidance
| Documentation | Description |
|---|---|
| Guideline on good pharmacovigilance practices (GVP) - Module VI – Collection, management and submission of reports of suspected adverse reactions to medicinal products (Rev. 2) | This module addresses the management and reporting of adverse reactions to medicinal products. |
| Guideline on good pharmacovigilance practices (GVP): Module IX – Signal management (Rev. 1) | This module describes the general guidance and requirements on structures and processes involved in signal management and how they are applied in the setting of the EU pharmacovigilance and regulatory network. |
Guidance documents
| Documentation | Description |
|---|---|
| (historical) |
|
| Note for guidance on the electronic data interchange (EDI) of individual case safety reports (ICSRS)1 and medicinal product reports (MPRS) in pharmacovigilance during the pre-and post-authorisation phase in the European ... |
|
| List of pharmaceutical dosage forms |
|
| E2B(R2) ICSR specification and related files | Technical ICH E2B(R2) ICSR message specification and related files |
| ICH Harmonised Tripartite Guideline - Maintenance of the ICH Guideline on Clinical Safety Data Management: Data Elements of Transmission of Individual Case Safety Reports E2B(R2) | The document provides details of the data elements for transmission of individual case safety reports by identifying and defining the data elements for the transmission of all types of individual case safety reports, regardless of the source and destination |
| E2B Implementation Working Group - Questions & Answers (R5) | This document provides questions and answers on the conventions for the harmonised interpretation of the ICH E2B(R2) guideline and messaging specifications |
Historical overview of EudraVigilance access policy revisions
| Documentation | Description |
|---|---|
| European Medicines Agency policy on access to EudraVigilance data for medicinal products for human use - Revision 3(historical) | The revised EudraVigilance access policy, which governs the level of access different stakeholder groups have to adverse drug reactions reports. The revised version came into force on 22 November 2017. This version was superseded by a new revision 4. |
| Overview of comments received on 'Draft revision of EudraVigilance access policy for medicines for human use' | The comments received on the draft revision of the EudraVigilance access policy during its public consultation between 4 August and 15 September 2014. |
| European Medicines Agency policy on access to EudraVigilance data for medicinal products for human use - Revision 2 (historical) | The revision 2 was adopted by EMA Management Board in December 2015. A minor technical update of Annex B has been carried out and this version was superseded by a new revision 3. |
| EudraVigilance access policy for medicines for human use(historical) | The EudraVigilance access policy governs the level of access different stakeholder groups have to adverse drug reactions reports. |
| Explanatory note: EudraVigilance access policy for medicines for human use | The explanatory note on the EudraVigilance access policy for medicines for human use published in July 2011 |
| Overview of comments received on draft EudraVigilance access policy and implemented amendments | The comments received on the draft EudraVigilance access policy during its public consultation and the implemented amendments published in July 2011 |
| Draft EudraVigilance access policy for medicines for human use | The draft EudraVigilance access policy for medicines for human use published in December 2008 |
Testing
Organisations preparing for electronic transmissions of ICSRs to EudraVigilance for the first time or performing major upgrades to established systems (e.g. submission of ICSRs in ICH E2B R3 format) should perform testing with EMA and consult EudraVigilance: electronic reporting for further information.
Guidance is available to support testing:
EMA published a question-and-answer document to address questions received from stakeholders in preparation of the launch of the new EudraVigilance system in 2017.
For more information, see:
The detailed change management plan provides stakeholders with information on the technical changes EMA is introducing in the EudraVigilance system as well as business process changes required to work with the enhanced system. EMA first published this document in October 2015 and the latest version of the plan in November 2017:
This provided a starting point for national competent authorities in the EEA, marketing authorisation holders and sponsors of clinical trials to develop their own internal implementation plans to prepare for the launch of the enhanced EudraVigilance system on 22 November 2017.
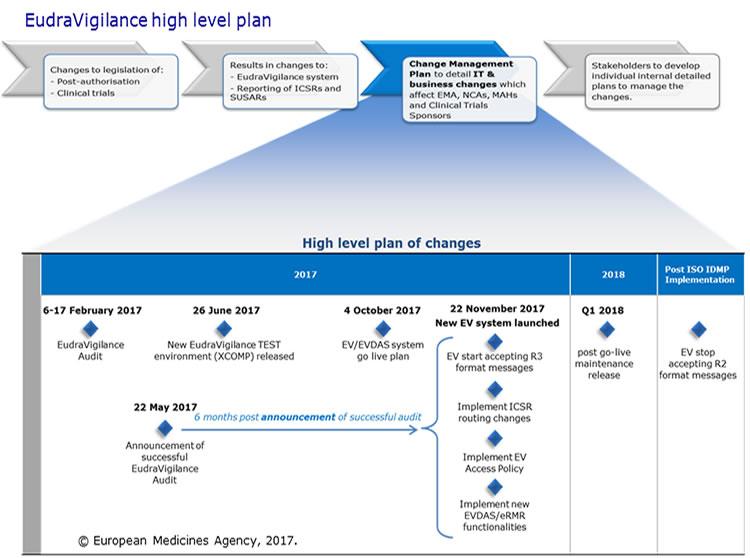
EudraVigilance high level plan
EudraVigilance go-live strategy
To enable EMA to release an enhanced Eudravigilance system on 22 November 2017, a downtime period of 10 business days was required. A number of key EudraVigilance functionalities were entirely or partially unavailable from 8 to 21 November 2017. Additional EMA IT systems were also affected during that period.
To facilitate the transition, the EU Regulatory network's pharmacovigilance business team prepared a EudraVigilance go-live plan and a technical note in consultation with the Pharmacovigilance Risk Assessment Committee (PRAC), the Clinical Trials Facilitation Group (CTFG) and the EudraVigilance Expert Working Group.
The plan outlined a set of detailed, sequenced tasks and activities, which were required prior to the enhanced EudraVigilance production system launch, explained the handling of legacy data resulting from the downtime and described the agreed temporary arrangements with each national competent authority.
The technical note described the IT systems that were affected by the downtime and provided further instructions to competent authorities, applicants, marketing authorisation holders and sponsors of clinical trials.
In addition, a The new EudraVigilance system – Public communications plan for European Medicines Agency and National Competent Authorities in the European Economic Area: overview of the planned public communication milestones described the EMA communication milestones with national competent authorities in the EEA that were part of the new EudraVigilance system go-live strategy. It identified the stakeholders affected, their needs and interests. Each Member State had the possibility to align its own communication strategy with the one prepared by EMA.
To enable EMA to release an enhanced Eudravigilance system on 22 November 2017, a downtime period of 10 business days was required. A number of key EudraVigilance functionalities were entirely or partially unavailable from 8 to 21 November 2017. Additional EMA IT systems were also affected during that period.
To facilitate the transition, the EU Regulatory network's pharmacovigilance business team prepared a EudraVigilance go-live plan and a technical note in consultation with the Pharmacovigilance Risk Assessment Committee (PRAC), the Clinical Trials Facilitation Group (CTFG) and the EudraVigilance Expert Working Group.
The plan outlined a set of detailed, sequenced tasks and activities, which were required prior to the enhanced EudraVigilance production system launch, explained the handling of legacy data resulting from the downtime and described the agreed temporary arrangements with each national competent authority.
The technical note described the IT systems that were affected by the downtime and provided further instructions to competent authorities, applicants, marketing authorisation holders and sponsors of clinical trials.
In addition, a The new EudraVigilance system – Public communications plan for European Medicines Agency and National Competent Authorities in the European Economic Area: overview of the planned public communication milestones described the EMA communication milestones with national competent authorities in the EEA that were part of the new EudraVigilance system go-live strategy. It identified the stakeholders affected, their needs and interests. Each Member State had the possibility to align its own communication strategy with the one prepared by EMA.