
Regulatory Science and Innovation
While addressing the immediate challenges of the COVID-19 pandemic, EMA continued with its efforts to ‘future-proof’ the Agency to ensure it is fit to tackle the scientific and technological challenges ahead and operates as efficiently as possible to deliver high-quality outputs for public and animal health.
Progress was made to fulfil the recommendations set in the Regulatory Science Strategy to 2025. Published in March 2020, this strategy aims at advancing regulatory science over the next five years, regarding both human and veterinary medicines. The strategy was developed together with the HMA as part of the ‘European medicines agencies network strategy to 2025’. This translated into specific actions and work planning spanning until 2025.
Actions linked to the regulatory science strategy started to take shape across the Agency. This includes the establishment of the ACT EU initiative, priority focus on novel manufacturing technique and advanced therapies and personalised medicines' developments, investment in real-world evidence capabilities, various digital innovation initiatives, as well as work done on health threats as part of the pandemic response. Progress made in these areas is described in other sections of this annual report as well as outreach to academia and research funders, which is described below.
Regulatory science research needs
In December 2021, EMA published the Regulatory Science Research Needs List, a list of regulatory science topics that need further research to close gaps and improve medicine development and evaluation to enable access to innovative medicines for patients. EMA identified around one hundred specific topics, in four categories relating both to human and veterinary medicines:
- integration of science and technology in medicine development;
- collaborative evidence generation to improve the scientific quality of evaluations;
- patient-centred access to medicines in partnership with healthcare systems;
- emerging health threats and availability/therapeutic challenges.
With this list, the Agency has started to stimulate researchers and funding organisations to address these topics in their research agendas and share their findings and results with regulators. By engaging in the Regulatory Science Research Needs initiative, researchers and sponsors will be able to see the findings translated into regulatory practice, medicine development and public health. The Agency's proactive exchanges with funding organisations and involvement in externally funded research projects also contribute to closing the regulatory science gaps.
Innovation Task Force support for 3R methodologies
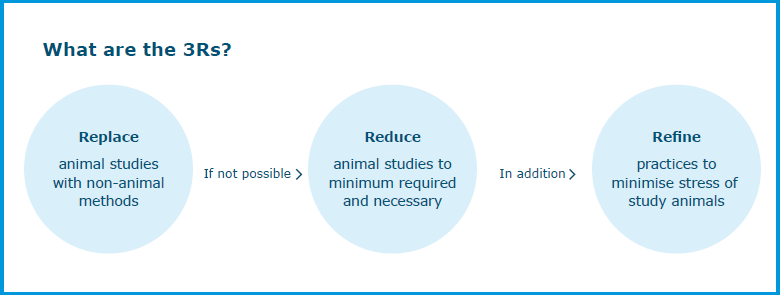
In September 2021, EMA opened up its Innovation Task Force (ITF) to the discussion of methodologies that minimise animal testing during medicine development. The ITF is a dedicated forum for early dialogue between regulators and developers of medicines to discuss innovative aspects such as emerging therapies, methods and technologies. The goal of this initiative is to facilitate the integration of the so-called 3Rs principles into the development and evaluation of medicinal products. The 3Rs are a set of principles supported by the Agency to replace, reduce and refine animal use for the development, manufacturing and testing of human and veterinary medicines.
This action facilitates the development and implementation of New Approach Methodologies that are in line with the European Union legislation on the protection of animals used for scientific purposes. It also supports a more adaptive regulatory system that will encourage innovation in human and veterinary medicine, as outlined in the Regulatory Science Strategy to 2025.
Borderline Classification Group
EMA and the HMA agreed in February 2021 the establishment of the Borderline Classification Group. This initiative, supported by the EU Innovation Network, aims to support a harmonised approach to the classification of innovative borderline products, i.e. products for which doubts arise as to whether they should be considered as medicinal products or fall under another regulatory framework, e.g. devices, food or cosmetics. This is to avoid situations where the same product could be classified differently depending on the approaches followed by each Member State, with consequences on the evidence requirements during development.
The Borderline Classification Group provides a multidisciplinary forum for informal discussions between competent authorities and other relevant groups at EU level, including the European Commission, to enable scientific and regulatory feedback to innovative complex drug developers with regards to the classification of their products and consequently the applicable legal/regulatory framework for these products and the evidence requirements. The first meeting of this group took place in March 2021.