
Regulation of veterinary medicines
Preparing for the implementation of the Veterinary Medicinal Products Regulation
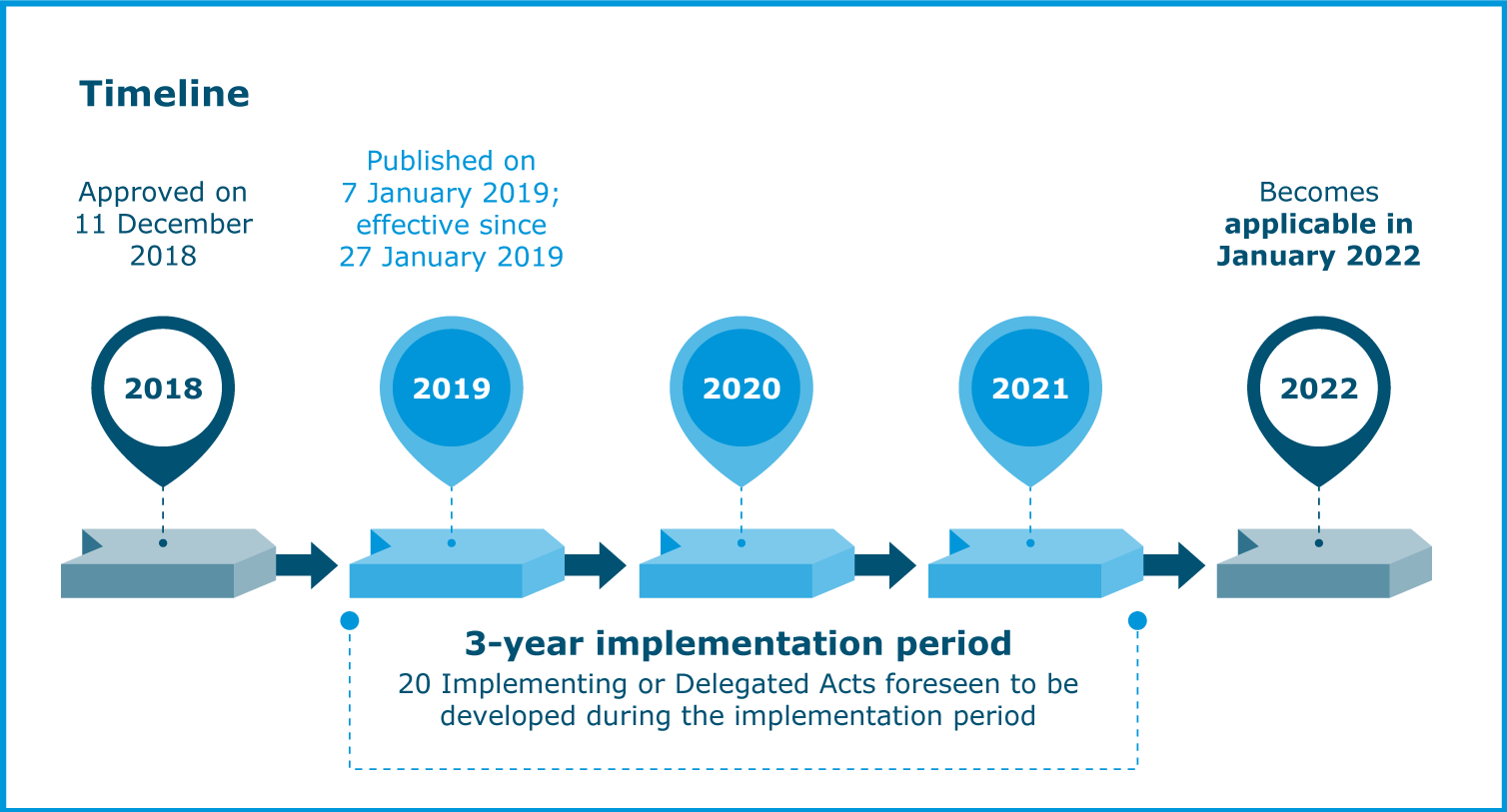
The implementation of the Veterinary Medicinal Products Regulation is the culmination of a process that started in 2018. EMA is responsible for coordinating and facilitating the work of the European medicines network on the implementation of the new legislation.
During the lead-up to the entering into application of the new Regulation, EMA revised procedures as well as regulatory and scientific guidance documents, to take account of the new rules. This was achieved through an open, constructive dialogue with the European Commission, national competent authorities and stakeholders on the practical implications of the new obligations and procedures. These regular meetings and exchanges with Member States and stakeholders also helped to prepare them for the changes brought by the Regulation at all levels.
2021 was a year in which the Agency reached many milestones that were needed for the new Regulation to become applicable on 28 January 2022.
Development of new processes and IT systems required by the Veterinary Medicinal Products Regulation
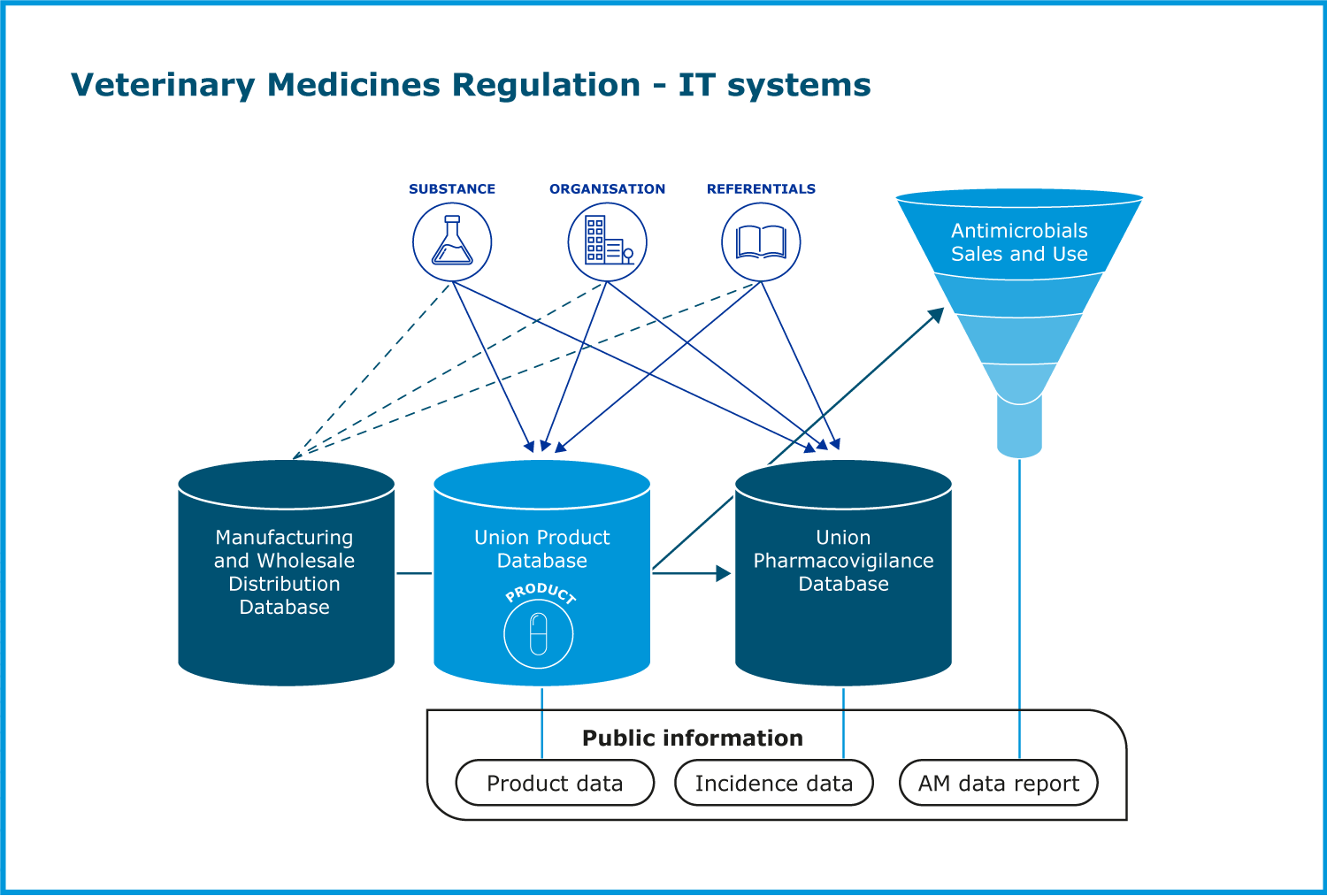
The Regulation calls for the establishment of a number of databases at EU level. EMA has led the development and implementation of the information technology systems required by the Regulation:
- Union Product Database (UPD)
- Union Pharmacovigilance Database (EVV)
- Manufacturing and Wholesale Distribution Database (MWD)
- Antimicrobials Sales and Use data (ASU)
The Union Product Database (UPD) project was established at the start of 2020 to ensure timely implementation of the requirements arising from the new Regulation. It gathers information on all veterinary medicines authorised in EU/EEA countries and will enable some post-authorisation procedures. One of the main priorities for 2021 was the submission of legacy data on veterinary medicinal products already authorised in the EU into the UPD which started during the summer. Timely delivery of the UPD components to enable submission was pivotal to achieve this goal. The Implementation Guide on veterinary medicines product data was finalised in June. After July, EMA’s efforts focused on providing support to national competent authorities (NCAs) for their upload of legacy data into the UPD. Weekly support sessions addressing practical questions and issues encountered while submitting data into the UPD were organised for all NCAs, as well as a tailored coaching programme to help NCAs to use the web user interface. By December 2021, 26,458 products had already been entered into the UPD. In parallel, the Agency developed the Veterinary Medicines information website, the public face of the UPD, which is the go-to source of information on all veterinary medicines in the EU/EEA.
The Union Pharmacovigilance Database (EVV) was developed as an enhanced and upgraded EudraVigilance Veterinary (EVVet3) system for the exchange and processing of suspected adverse reaction reports related to veterinary medicines authorised in the EEA. In January 2021, the Product Owners Group was expanded to include representatives from the veterinary pharmaceutical industry, ensuring that the views and needs of all relevant stakeholders are represented. An access policy was presented during the June 2021 meeting of the EMA Management Board and subsequently published on the EMA website. In September, the Project Group approved the final requirements for the recording of pharmacovigilance inspection outcomes and for signal management.
Development of the Manufacturers and Wholesale Distributors database (MWD) started in July 2021 after the approval of the project vision and the finalisation of the detailed requirements. Most of the legislative requirements outlined by the new Regulation were covered by the existing EudraGMDP system, the EU database of manufacturing authorisations and certificates of good manufacturing practice. However, the existing system required some functionality extensions for full compliance. Four modules of EudraGMDP were impacted. EMA organised a first demonstration of the system under development to NCAs in September 2021. Webinars were held in October 2021 to support preparedness of NCAs and industry.
The new legislation also puts in place a range of measures to limit the development of AMR. At the heart of these efforts is the Collection of Antimicrobial Sales and Use data (ASU) project, which started in 2021. The ASU Project Group approved the final business case, project vision and implementation timeline throughout the year, and started working on gathering detailed requirements. In December, EMA and the Collection of ASU Project Group introduced the project to the members of the European Surveillance of Veterinary Antimicrobial Consumption (ESVAC) network. This was the first of a series of meetings to update NCAs on the project progress and prepare them for the data submission expected to start in 2023. The Agency also published guidance and Q&As for marketing authorisation holders on how to submit data on the volume of sales.
Veterinary limited markets
In March 2021, EMA released the eleventh report on the MUMS and limited markets scheme for veterinary medicines. The report concluded that the scheme has been successful in terms of incentivising the submission of requests for classification of products as MUMS/limited market, resulting in new veterinary medicines becoming available to treat minor animal species and uncommon diseases in major animal species. Building on the success of EMA’s experience applying this policy over the last ten years, the new veterinary regulation introduced specific provisions in the EU legislation to boost the development of new medicines for limited markets. In 2021, EMA issued several guidelines to replace those applicable under EMA's MUMS/limited market policy and to help applicants to consider the criteria for eligibility of products for a limited market authorisation and benefit from reduced data requirements introduced by the new Regulation.
A new international standard format for reporting adverse events
With the implementation of the Union Pharmacovigilance Database, the data format previously used has been replaced by the pharmacovigilance reporting standards developed by the Veterinary International Conference on Harmonization (VICH). In October 2021, EMA released the EU adverse event report (VICH) implementation guide. This document aims to support stakeholders in the implementation of the Regulation on veterinary medicinal products, by providing guidance on the technical specifications and the process of transmission of adverse event reports (AERs). It is targeted at all stakeholders responsible for submitting AERs electronically to the Union Pharmacovigilance Database. It describes the rules that stakeholders must follow to ensure successful electronic communication between their own systems and EudraVigilance Veterinary (EVV).