
Regulatory science strategy to 2025
PREPARING FOR THE FUTURE – REGULATORY SCIENCE TO 2025
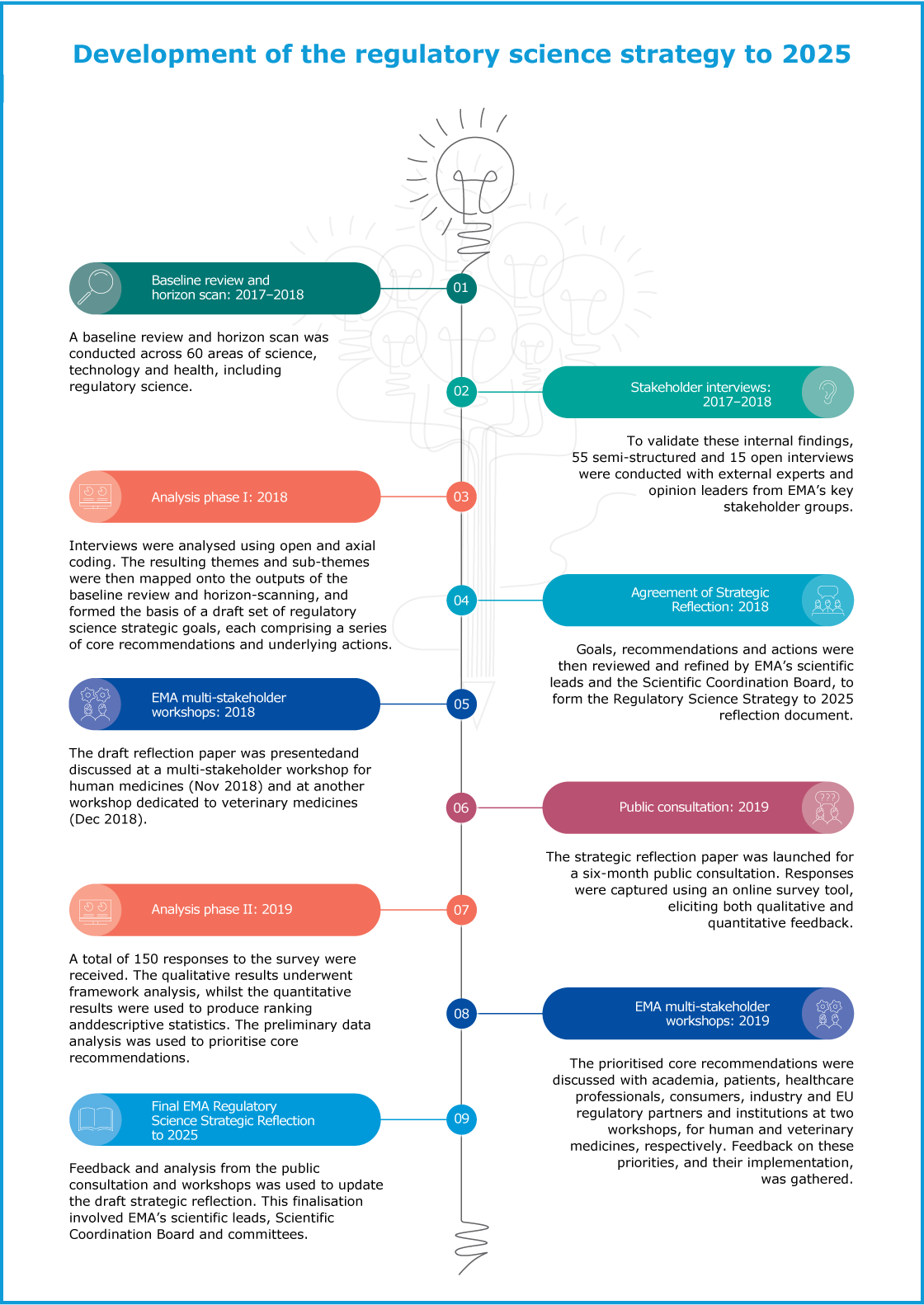
Regulatory science is at the foundation of everything that EMA does to make medicines available for the benefit of public and animal health. EMA supports the development of regulatory science and aims to ensure that advances in knowledge translate in a timely way into new, safe and effective treatments for patients and animals. In 2019, a priority for EMA was to shape its plan for advancing regulatory science over the next five to ten years, in both human and veterinary medicines.
GROUNDWORK LAID FOR THE REGULATORY SCIENCE STRATEGY TO 2025
Following a dynamic period of reflection on scientific and technological advances in the pharmaceutical arena and the future challenges EMA’s scientific committees and working parties will face due to these developments, the Agency focused on building consensus on future priorities and resource allocation.
The Regulatory Science Strategy is the most comprehensive reflection we have conducted as an Agency since its origin. We have reflected on how we are going to engage with various challenges that new science and technology present in terms of medicines regulation, not only in Europe but also globally.
This image is taken from: Philip A. Hines, Rosanne Janssens, Rosa Gonzalez-Quevedo, Apolline I.O.M. Lambert, Anthony J. Humphreys; A future for regulatory science in the European Union: the European Medicines Agency’s strategy, Nature Review Drug Discovery, Comment 31 March 2020. (doi:10.1038/d41573-020-00032-0)
SEEKING STAKEHOLDERS’ VIEWS
In the first half of 2019, EMA completed an extensive public consultation process to refine and prioritise key areas. The Agency reached out to a wide range of public health stakeholders and experts at all levels of medicine development, including patients, healthcare professionals, pharmaceutical industry, academia, and other regulatory bodies.
The purpose of the public consultation was to seek the widest possible views on whether the proposed core recommendations and supporting actions address stakeholders’ needs.
The Regulatory Science Strategy comes at the right time. As we implement the new Veterinary Medicines Regulation, it can support us in developing a science-based regulatory tool-box to improve the evaluation of benefits and risks of veterinary medicines.
OUTCOME OF PUBLIC CONSULTATION
EMA received constructive feedback on its draft strategy from over 150 respondents from a broad range of stakeholder groups, including academia, healthcare professionals, and the pharmaceutical industry that participated in this exercise and provided comments. Stakeholders used the opportunity to help guide the future strategic application of financial and human resources to address the greatest needs. The figure below shows an overview of stakeholder views on which areas required prioritisation.
STAKEHOLDER VIEWS ON WHICH AREAS REQUIRE PRIORITISATION
Overall, stakeholders found the draft regulatory science strategy comprehensive and recognised that it addresses relevant issues. They stressed the importance of stakeholder engagement for its implementation and acknowledged the Agency’s efforts to collaborate with the wider stakeholder community, including patients, healthcare professionals, the pharmaceutical industry, other regulators and health technology assessment bodies and payers. All inputs from the public consultation, strategic reflections and discussions will be distilled into a comprehensive document that will be published in early 2020.
STRATEGIC REFLECTIONS ON THE REGULATORY SCIENCE STRATEGY TO 2025
Following the public consultation, the Agency hosted two multi-stakeholder workshops on human and veterinary medicines in November and December 2019. The meetings served to reach agreement on key areas where changes are required in the coming years. The finalised strategy post consultation will be a key element of the next European Regulatory Network Strategy to 2025. This will be developed together with the Member States and the European Commission and will feed directly into the Agency’s multiannual work programme and the work plans of its committees and working parties.

For this annual report, EMA has asked its own experts and stakeholders for their views on the development of the Regulatory Science Strategy.
Expert views on the development of the Regulatory Science Strategy
Anthony Humphreys, Head of Regulatory Science and Innovation Task Force
WHAT ARE THE OUTCOMES OF THE PUBLIC CONSULTATION ON THE REGULATORY SCIENCE STRATEGY TO 2025?
We were extremely pleased with the diversity of engagement across all of our various stakeholder groups. We received substantial input from healthcare professionals, patient groups, academia, industry, other EU institutions, NCAs and downstream decision-makers. That has allowed us to converge on what are seen as the most significant changes we could make as a system over the next five years that would deliver real value to improve the regulation of medicines in Europe.
WHAT ARE THE KEY TAKEAWAYS FROM THE MULTI-STAKEHOLDER DISCUSSIONS?
When digesting all the feedback we received from stakeholders, we converged on a number of very important points: fostering innovation and clinical trials; looking into real-world evidence, real-world data sources and how they can be used in benefitrisk decision-making; and trying to increase patient involvement throughout the whole development life cycle to ensure the medicines being delivered meet patient needs and expectations. We have also recognised that we can leverage and collaborate with our academic colleagues better to help us address regulatory challenges. We must also engage with the challenges of the new biology and personalised precision medicines. It is a very exciting time as regards the potential this offers patients, but it is also very challenging. How do we really engage with the extent of this interesting, challenging science that is coming through? That’s at the very heart of this reflection on regulatory science.
WHAT ARE THE MOST IMPORTANT LESSONS LEARNT FROM THE PUBLIC CONSULTATION?
Having benefitted from the very extensive sixmonth consultation process, we really were in a listening mode and willing to receive input from our stakeholders, to adapt what we will focus on in the future, and to deliver what we anticipate to be the greatest changes needed to ensure the most impact.
WHO ARE THE KEY ACTORS WHO NEED TO BE INVOLVED IN IMPLEMENTING THE ACTIONS OUTLINED IN THE RSS?
Having listened to our stakeholders and agreed on a common set of priorities and underlying actions to deliver change in a given area, we have to work together to try to make it happen. It is this spirit of collaboration and cooperation as we go forward that is at the very heart of the exercise. It is going to be one of the most critical success factors to deliver the strategy.
CONSIDERING THE PROGRESS MADE IN 2019, WHAT ARE THE NEXT STEPS?
We held major workshops at the end of 2018 and, after a very extensive public consultation, we drew that reflection to a close in the last quarter of 2019 with both human and veterinary workshops. We are now writing up all that valuable feedback with a view to formal adoption by our Management Board in March 2020. At the same time, this regulatory science reflection is not a stand-alone exercise. Many critical changes will have to be delivered together with our network partners as part of the EU Network Strategy to 2025 which we are now actively putting together with the heads of agencies of the NCAs.
HOW WILL THE REGULATORY SCIENCE STRATEGY BE IMPLEMENTED?
The Regulatory Science Strategy underlines the need for EMA experts and staff to keep pace with technological and scientific developments in order to assess innovative and increasingly complex medicines that are intersecting many different technologies. Throughout 2019, as we developed the strategy, we realised that our current organisation would need to be modified to meet these future challenges. The primary outcome of that future-proofing exercise has been the consolidation of the three human medicines divisions into a single division. This will focus on delivering the necessary changes in maintaining and improving the core business, together with the constitution of four task forces on digital business transformation, data analytics and methods, regulatory science and innovation, and clinical trials and manufacturing strategy. At the heart of their origin is delivering on the changes needed on one or more of the key strategic areas of the Regulatory Science Strategy.

Tjalling van der Schors, hospital pharmacist, board member at the European Association of Hospital Pharmacists (EAHP), Director of Professional Development
The Regulatory Science Strategy 2025 is well designed to reach the very-much-needed changes to ensure that medicines of the future will be safe and effective. The complexity requires difficult choices in the balance between evidence and progress. Patients need reliable medicines. The evidence to achieve this should not result in an unwanted increase of administrative burden for healthcare professionals.

Thomas Kanga-Tona, project manager at the International Association of Mutual Benefit Societies (AIM)
What does the Regulatory Science Strategy to 2025 mean to you?
Overall, AIM subscribes to the EMA strategy and welcomes the fact that the Regulatory Science Strategy to 2025 highlights the need for closer collaboration/engagement with payer organisations and their needs. EMA’s Regulatory Science Strategy is the opportunity for the Agency to clearly state its priorities for the next five years, with regards to its core tasks: making sure that safe, quality and efficacious medicines reach European markets.
What is the importance of the Regulatory Science Strategy to 2025 for health technology assessment bodies and payers?
For AIM, as a payers’ organisation, the Regulatory Science Strategy to 2025 is the opportunity to provide feedback to the regulator about our insights on key trends we have noticed in recent years. We think that EMA should ensure there is strong data evidence at the time of marketing approval. It would help reduce uncertainty and allow for more timely access as it could improve the relevance of the end points. We would very much welcome EMA taking into account the concerns AIM has already raised about the accelerated pathways for drug approval. If more data needs to be collected after market access, EMA should be more stringent regarding the follow-up of requirements set for companies against the background of increasing the relevance of end points and ensuring that only safe and effective medicines are used in the EU. In general, when evaluating new technologies (especially ATMPs) we would like to ask EMA to also consider the impact on the healthcare setting/patient treatment process as a whole. Situations where technologies would only be available in certain centres in Europe, and fragile patients (and their families) would need to travel far from home and stay in foreign hospitals for months might have an important impact for the patient outcomes (including quality of life).
What did you think of the consultation process?
AIM replied to the public consultation and attended the community event which took place in November. However, we have heard little so far about how the final strategy will look, which topics will feature in it, as well as how they will be chosen. The overall timeline for the adoption of the strategy is quite opaque, too.

Anton Ussi, Operations and Finance Director of the European Infrastructure for Translational Medicine (EATRIS)
What does the Regulatory Science Strategy to 2025 mean to you?
We are very optimistic about both the strategy and opportunities it affords the academic community. Recognition of the ever-increasing complexity of health innovations calls for a multi-stakeholder effort and structural interaction among these. The strategy has put this need front and centre, and deeper collaboration with academia will be an important element of this.
What is the importance of the Regulatory Science Strategy to 2025 for academia?
While academia has always been a pillar of the innovation process, we see academics playing a central role in innovation more often and going further downstream towards clinical application more often than ever before, especially in advanced therapies. As a community, we need to be able to understand from the regulator what is expected of us, as well as to be able to share our knowledge of these emerging technologies so that regulatory clarity can be achieved quickly. The strategy recognises that this two-way communication is essential and charts a clear path to facilitate it.
What do you think of the consultation process?
The consultation was open, transparent and extremely well executed. We were given the opportunity to provide in-depth responses, and the EMA was very active in ensuring that the consultation was broadly disseminated and utilised. After collation, the face-to-face workshop was deeply satisfying as we had a genuine sense of openness, and the feeling that the Agency is in listening mode. This bodes well for the future of the strategy. Hats off to the Agency for continuing apace with these developments in the midst of making an historical transition to Amsterdam.

Jean-Pierre Orand, Director of the French Agency for Veterinary Medicines (ANMV)
What does the Regulatory Science Strategy to 2025 mean to you?
The Regulatory Science Strategy is an opportunity to reinforce the network, especially the relationship between regulator and academia, and to answer to the challenges we are confronted with. The European medicines network of agencies has to be prepared for future problems of animal and human health. One of the regulator’s strategic objectives is to adapt the available scientific and regulatory expertise both in terms of capacity and capability to cope with changing demands. We will develop the capability to regulate novel products of the future, consider greater use of real-world databases and increase transparency about the data that underpin regulatory decisions.
Regulatory science will promote innovation to respond to therapeutic gap and be a tool to facilitate the registration of novel and innovative medicines, taking into account the specificities of the veterinary sector and considering the social and economic context.
So, the Regulatory Science Strategy to 2025 is a way to prepare for these challenges for the benefit of human and animal health.
What is the importance of the Regulatory Science Strategy to 2025 for veterinarians?
Animal health has a direct impact on human health; it is the concept of the One Health approach. Most of the diseases are zoonosis, and animals remain the principal source of protein for human consumption.
At the same time, the veterinary medicines sector faces different constraints compared to the human medicines sector. On one hand, there are many therapeutic gaps due to the high number of species and lots of minor use. On the other hand, the development of veterinary medicines will take into account economical aspects with the need for return of investment and low prices, environmental risk management, societal consideration (animal welfare, etc.) and an increase in animal health outbreaks in the world due to the globalisation of the market and the increasing circulation of people and goods, and climate change.
So, regulatory science might bring answers to all these constraints by developing another approach for increasing the availability of veterinary medicines, not only through innovation but also by developing new approaches for improving the benefit-risk assessment or application of the latest scientific knowledge.
A new legal framework has just been adopted for veterinary medicines and with the Regulatory Science Strategy there is an opportunity to develop a better environment for the authorisation of veterinary medicines.
What did you think of the Regulatory Science Strategy consultation process?
The consultation process was very interesting as lots of stakeholders had a chance to share their views with transparency and openness. The face-to-face workshop organised after the written phase was really satisfying as it was an opportunity to have fruitful exchanges with all stakeholders and to clarify certain opinions or ideas that came up during the written phase.

Nancy De Briyne, Deputy Executive Director of the Federation of Veterinarians of Europe (FVE)
What does the Regulatory Science Strategy to 2025 mean to you?
The Regulatory Science Strategy for me is a vision and strategy document. It shows the road that legislators in the field of veterinary medicine need to take to keep up with rapidly changing societal and technological developments. It aims to make sure that health professionals will be able to continue to have the best medicines available to treat their patients and that we are prepared for emerging health threats.
“The best way to predict the future is to create it.” (Lincoln)
What is the importance of the Regulatory Science Strategy to 2025 to veterinarians?
The availability of effective, safe and affordable veterinary medicines is extremely important for veterinary surgeons to safeguard the health and welfare of their patients. Many of the issues impacting the daily work of veterinarians are covered in this strategy, e.g. how to regulate innovative medicines such as immunomodulators and phages; how to ensure the availability of medicines for all animals and in all EU countries; how to make the best use of precision medicine; how to control AMR; and how to prepare for emerging diseases due to climate change. All these important topics are dealt with in the strategy.
What did you think of the Regulatory Science Strategy consultation process?
EMA did not try to rush the exercise but took extensive time to consult widely with all the stakeholders involved. FVE had an ample opportunity to collect the views of European veterinarians, attended several consultation meetings and gave input at several time frames. As such, FVE very much appreciates the consultation process.

