Hero image
Image

Pre heading
CHAPTER 2 - KEY FIGURES IN 2019
Human medicines
Assembly area
Heading
Rich text
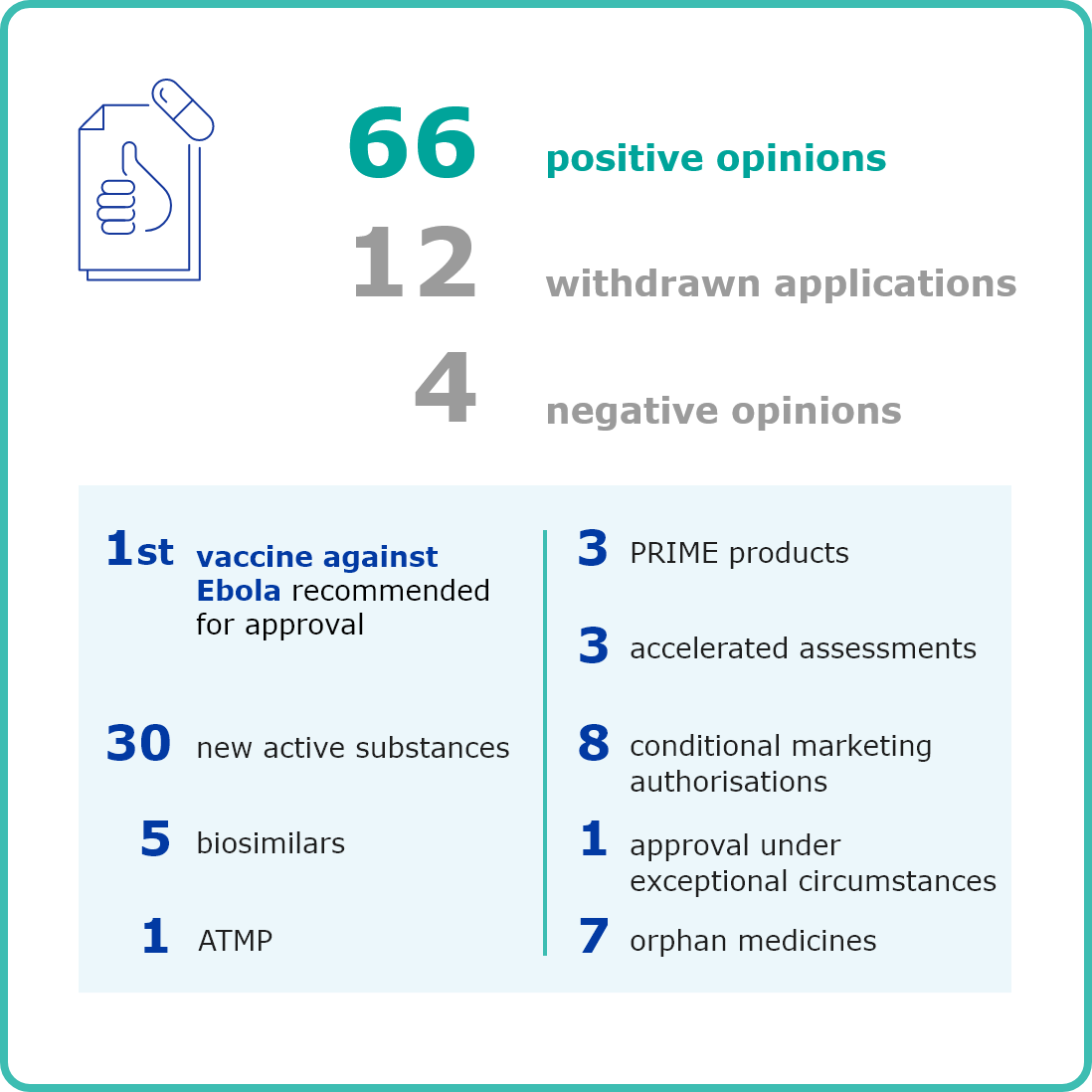
EMA is responsible for the scientific evaluation, supervision and safety monitoring of medicines in the EU. In 2019, EMA received a record number of requests for scientific advice. The numbers of applications for marketing authorisation were up again and EMA recommended 66 new medicines for marketing authorisation. EMA continued to closely monitor the safety of medicines on the market and take action when needed. The product information for 405 centrally authorised medicines was updated on the basis of new safety data. Here are some key figures on the authorisation and safety monitoring of medicines for human use in 2019. For more detailed information, download the full annual report 2019 (PDF version).
Heading
Rich text
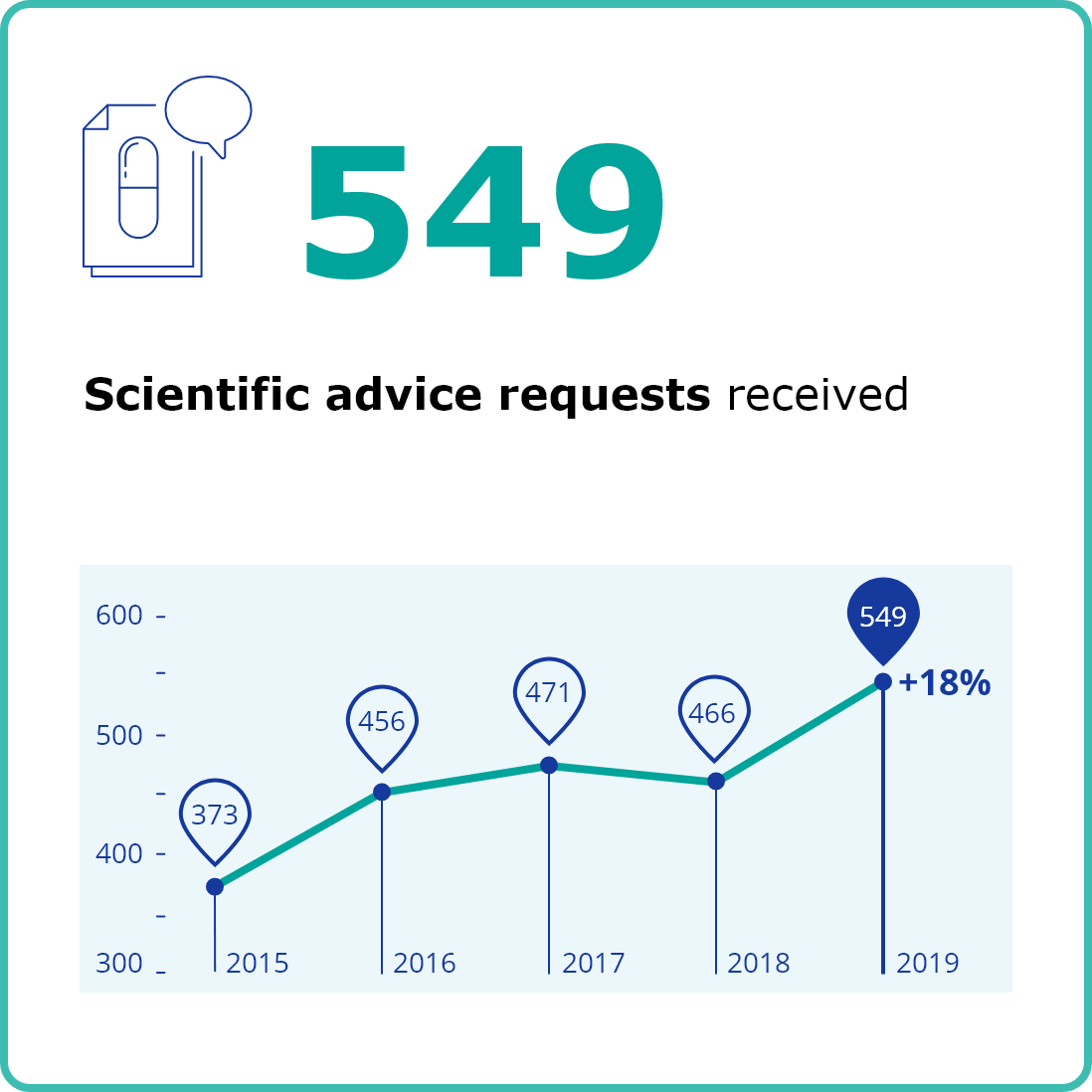
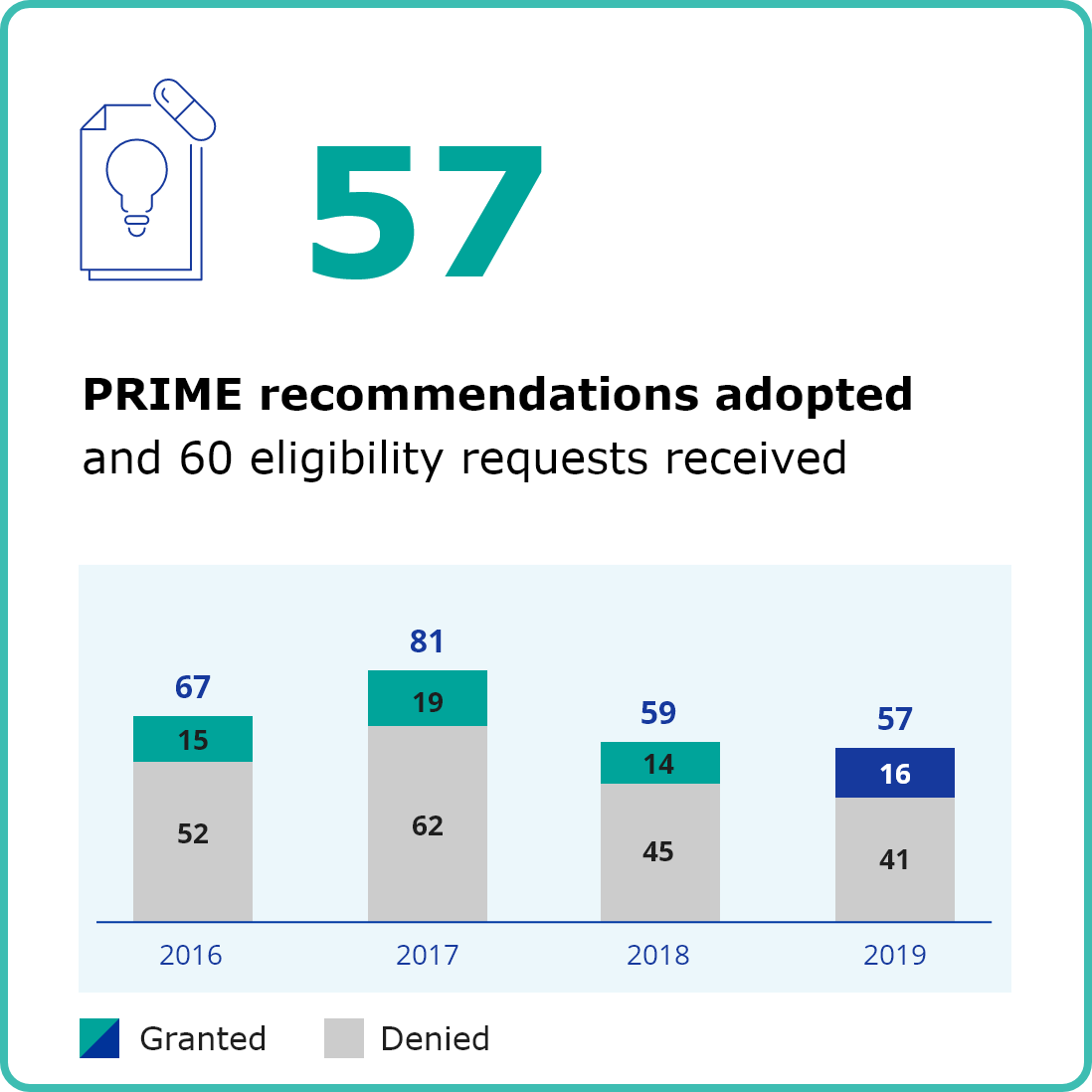
SUPPORTING RESEARCH AND DEVELOPMENT
First column content
Image
Second column content
Image
Heading
Rich text
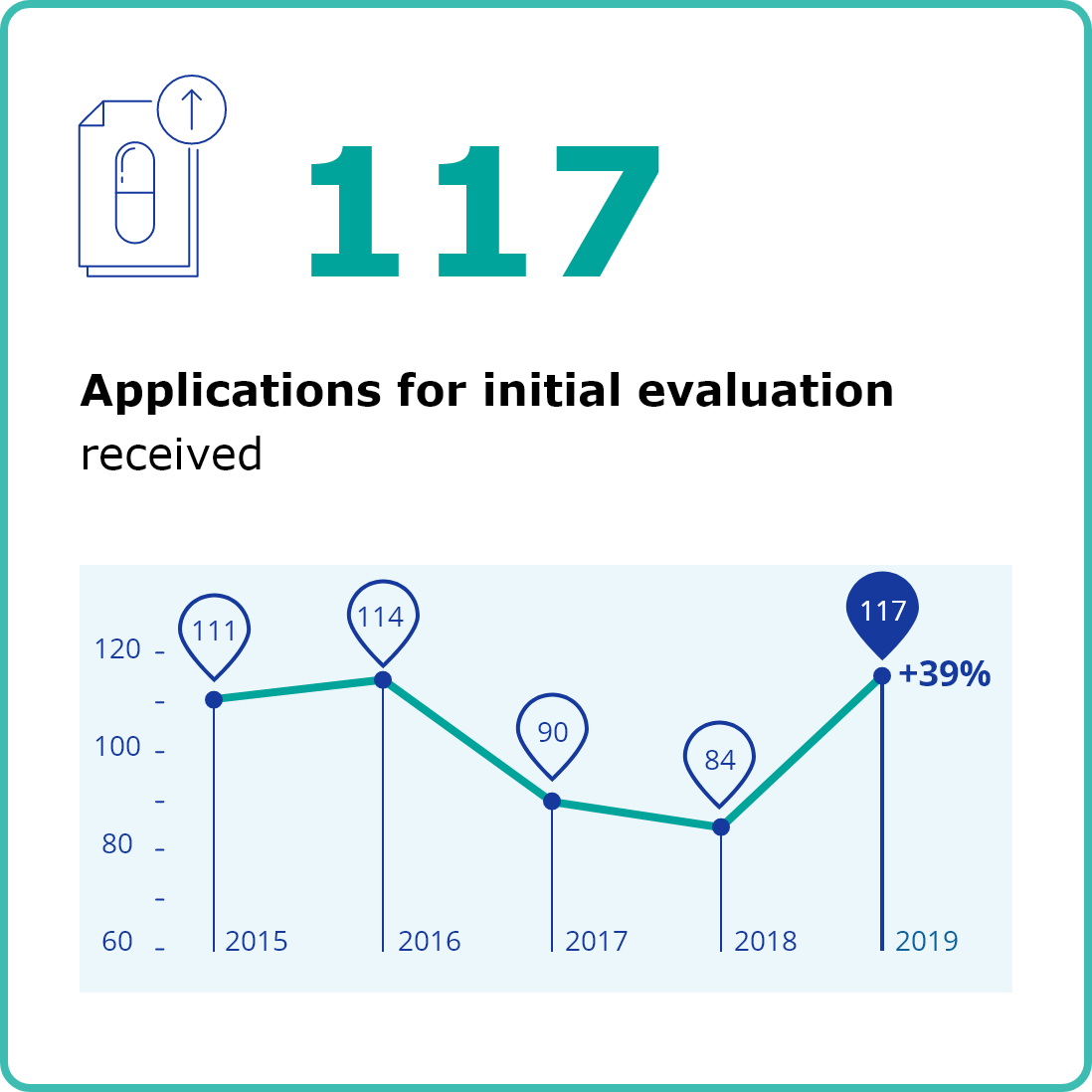
RECOMMENDATIONS FOR MARKETING AUTHORISATION
First column content
Image
Second column content
Image
Heading
Rich text
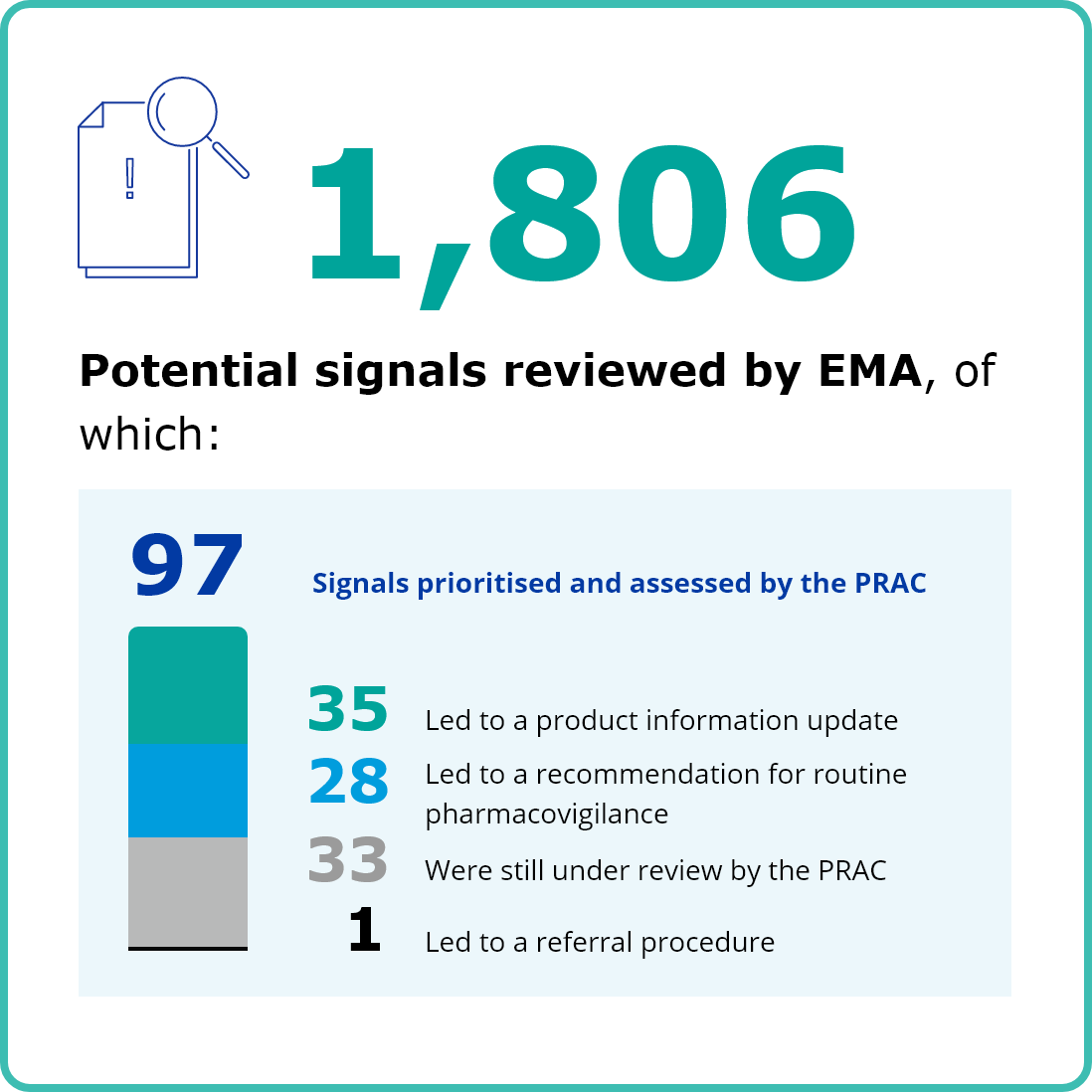
SAFETY MONITORING OF MEDICINES
First column content
Image
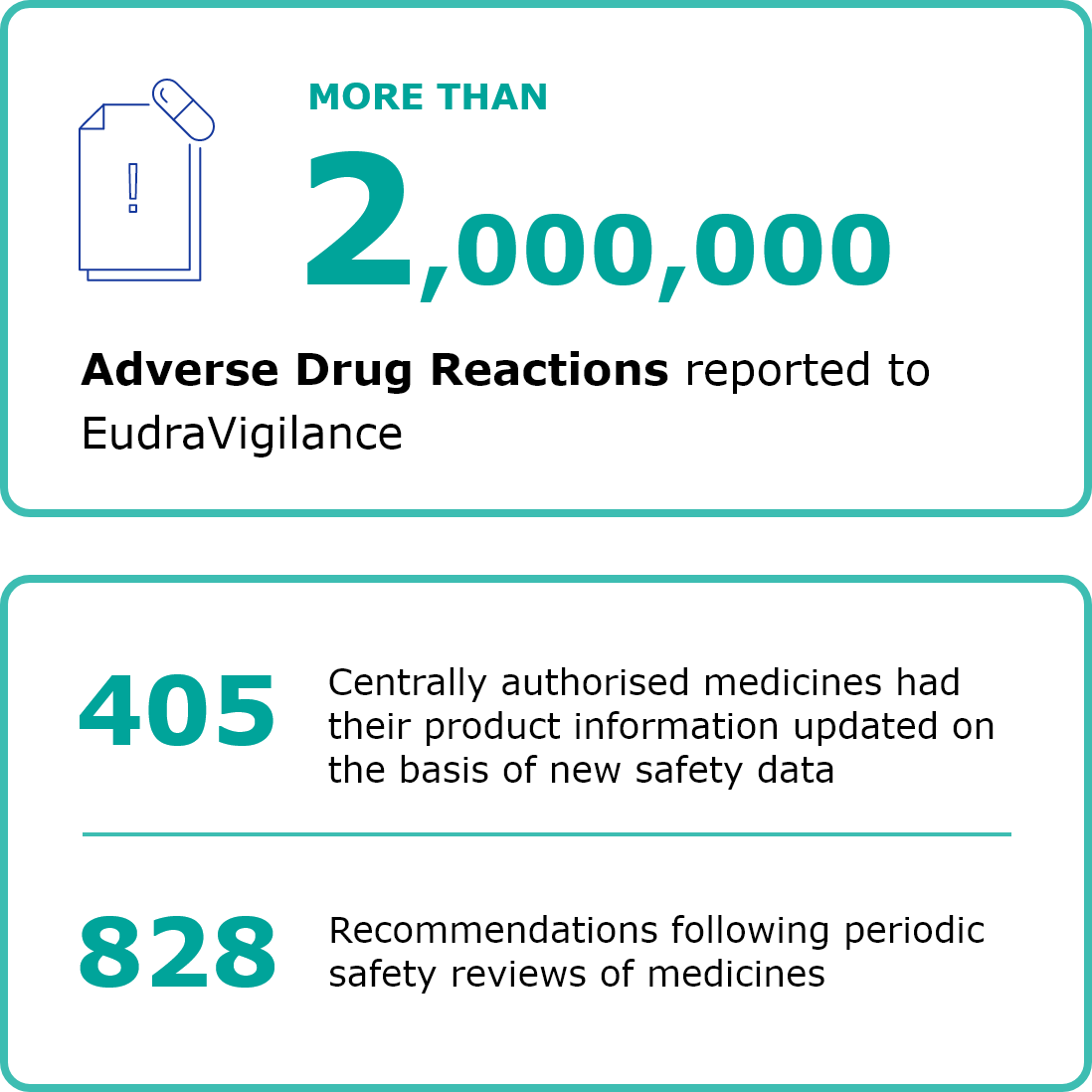
Second column content
Image