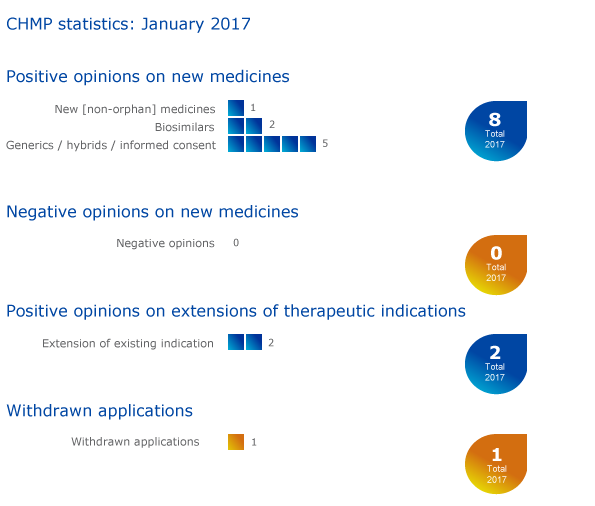
Eight medicines recommended for approval, including two biosimilars
The European Medicines Agency's Committee for Medicinal Products for Human Use (CHMP) recommended eight medicines for approval at its January meeting.
The Committee recommended granting a marketing authorisation for Xeljanz (tofacitinib) for the treatment of rheumatoid arthritis.
One hybrid medicine, Jylamvo (methotrexate) received a positive opinion for the treatment of rheumatological disorders and psoriasis, and for the maintenance treatment of acute lymphoblastic leukaemia (ALL). Hybrid applications rely in part on the results of pre-clinical tests and clinical trials for a reference product and in part on new data.
Two biosimilar medicines were recommended for approval by the Committee: Amgevita and Solymbic, both containing adalimumab. Amgevita is for the treatment of rheumatoid arthritis, juvenile idiopathic arthritis, axial spondyloarthritis, psoriatic arthritis, psoriasis, paediatric plaque psoriasis, hidradenitis suppurativa, Crohn's disease, paediatric Crohn's disease, ulcerative colitis and uveitis. Solymbic is intended for the treatment of rheumatoid arthritis, enthesitis-related arthritis, axial spondyloarthritis, psoriatic arthritis, psoriasis, hidradenitis suppurativa, Crohn's disease, ulcerative colitis and uveitis. A biosimilar medicine is a biological medicine that is highly similar to another biological medicine that is already authorised for use.
The CHMP granted positive opinions for two informed consent applications: Rolufta (umeclidinium) for the treatment of chronic obstructive pulmonary disease (COPD) and Tadalafil Lilly (tadalafil) for the treatment of erectile dysfunction and the signs and symptoms of benign prostatic hyperplasia. An informed consent application makes use of data from the dossier of a previously authorised medicine, with the marketing authorisation holder of that medicine giving consent for the use of their data in the application.
Two generic medicines received a positive opinion from the Committee: Yargesa (miglustat) for the treatment of Gaucher disease and Daptomycin Hospira (daptomycin) for the treatment of complicated skin and soft-tissue infections (cSSTI), right-sided infective endocarditis (RIE) due to Staphylococcus aureus and S. aureus bacteraemia associated with RIE or with cSSTI.
Two recommendations on extensions of therapeutic indications
The Committee recommended extensions of indications for Revlimid and Synjardy.
Withdrawals of applications
The application for a marketing authorisation for Zioxtenzo (pegfilgrastim) has been withdrawn. Zioxtenzo was developed as a biosimilar medicine to treat neutropenia in cancer patients.
An application to extend the indication of Xgeva (denosumab) to treat hypercalcemia of malignancy (high levels of calcium in the blood caused by cancer) has also been withdrawn.
Questions-and-answers documents on these withdrawals are available in the grid below.
Outcome of review on medicines containing dienogest and ethinylestradiol
The CHMP has recommended that medicines containing a combination of dienogest 2 mg and ethinylestradiol 0.03 mg can continue to be used to treat moderate acne when suitable treatments applied to the skin or antibiotics taken by mouth have not worked. However, these medicines, which are also approved as hormonal contraceptives, should only be used in women who choose oral contraception. For more information please see the public health communication in the grid below.
Agenda and minutes
The agenda of the January 2017 meeting is published on EMA's website. Minutes of the December 2016 CHMP meeting will be published next week.
CHMP statistics
Key figures from the January 2017 CHMP meeting are represented in the graphic below.
More information on this, and all other outcomes of the CHMP's January 2017 meeting, is available in the grid below.
Positive recommendation on new medicine
| Name of medicine | Xeljanz |
|---|---|
| INN | tofacitinib |
| Marketing-authorisation applicant | Pfizer Limited |
| Therapeutic indication | Treatment of rheumatoid arthritis |
| More information | CHMP summary of positive opinion for Xeljanz |
Positive recommendations on new informed-consent applications
| Name of medicine | Rolufta |
|---|---|
| INN | umeclidinium |
| Marketing-authorisation applicant | GlaxoSmithKline Trading Services |
| Therapeutic indication | Treatment of chronic obstructive pulmonary disease (COPD) |
| More information | CHMP summary of positive opinion for Rolufta |
| Name of medicine | Tadalafil Lilly |
|---|---|
| INN | tadalafil |
| Marketing-authorisation applicant | Eli Lilly Nederland B.V. |
| Therapeutic indication | Treatment of erectile dysfunction in adult males and treatment of the signs and symptoms of benign prostatic hyperplasia |
| More information | CHMP summary of positive opinion for Tadalafil Lilly |
Positive recommendations on new generic medicines
| Name of medicine | Daptomycin Hospira |
|---|---|
| INN | daptomycin |
| Marketing-authorisation applicant | Hospira UK Limited |
| Therapeutic indication | Treatment of complicated skin and soft tissue infections (cSSTI), right-sided infective endocarditis (RIE) due to Staphylococcus aureus and S. aureus bacteraemia associated with RIE or with cSSTI |
| More information | CHMP summary of positive opinion for Daptomycin Hospira |
| Name of medicine | Yargesa |
|---|---|
| INN | miglustat |
| Marketing-authorisation applicant | JensonR+ Limited |
| Therapeutic indication | Treatment of Gaucher disease |
| More information | CHMP summary of positive opinion for Yargesa |
Positive recommendation on new hybrid medicine
| Name of medicine | Jylamvo |
|---|---|
| INN | methotrexate |
| Marketing-authorisation applicant | Therakind Limited |
| Therapeutic indication | Treatment of rheumatological disorders and psoriasis and for the maintenance treatment of acute lymphoblastic leukaemia (ALL) |
| More information | CHMP summary of positive opinion for Jylamvo |
Positive recommendations on new biosimilar medicines
| Name of medicine | Amgevita |
|---|---|
| INN | adalimumab |
| Marketing-authorisation applicant | Amgen Europe B.V. |
| Therapeutic indication | Treatment of rheumatoid arthritis, juvenile idiopathic arthritis, axial spondyloarthritis, psoriatic arthritis, psoriasis, hidradenitis suppurativa, Crohn's disease, ulcerative colitis and uveitis |
| More information | CHMP summary of positive opinion for Amgevita |
| Name of medicine | Solymbic |
|---|---|
| INN | adalimumab |
| Marketing-authorisation applicant | Amgen Europe B.V. |
| Therapeutic indication | Treatment of rheumatoid arthritis, enthesitis-related arthritis, axial spondyloarthritis, psoriatic arthritis, psoriasis, hidradenitis suppurativa, Crohn's disease, ulcerative colitis and uveitis |
| More information |
Positive recommendations on extensions of therapeutic indications
| Name of medicine | Revlimid |
|---|---|
| INN | lenalidomide |
| Marketing-authorisation holder | Celgene Europe Limited |
| More information | CHMP post-authorisation summary of positive opinion for Revlimid (II-89G) |
| Name of medicine | Synjardy |
|---|---|
| INN | empagliflozin / metformin |
| Marketing-authorisation holder | Boehringer Ingelheim GmbH |
| More information | CHMP post-authorisation summary of positive opinion for Synjardy |
Withdrawals of applications
| Name of medicine | Xgeva |
|---|---|
| INN | denosumab |
| Marketing-authorisation holder | Amgen Europe B.V. |
| More information | Questions and answers on the withdrawal of the marketing authorisation application for Xgeva (denosumab) |
| Name of medicine | Zioxtenzo |
|---|---|
| INN | pegfilgrastim |
| Marketing-authorisation holder | Sandoz GmbH |
| More information | Questions and answers on the withdrawal of the marketing authorisation application for Zioxtenzo (pegfilgrastim) |
Public health recommendation
| Name of medicine | Dienogest / Ethinylestradiol |
|---|---|
| INN | dienogest / ethinylestradiol |
| More information | Dienogest/ethinylestradiol can be used for acne after certain other treatments have failed |
Other updates