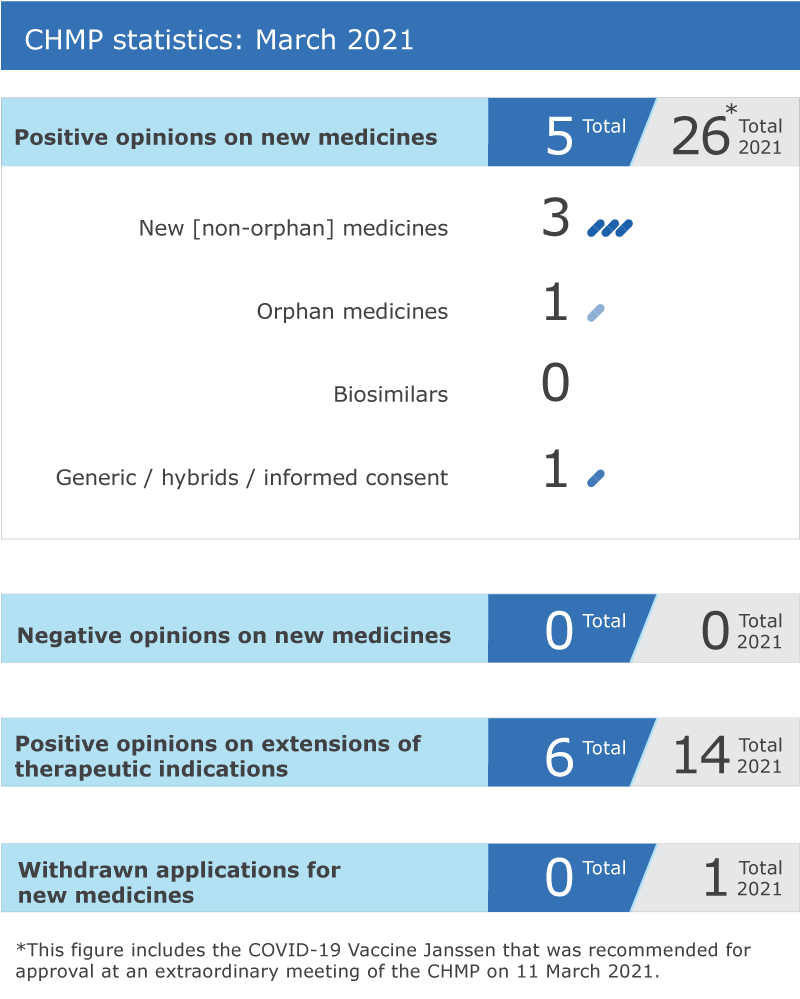
Five new medicines recommended for approval
EMA’s human medicines committee (CHMP) recommended five medicines for approval at its March 2021 meeting.
The Committee recommended granting a marketing authorisation for Copiktra (duvelisib) for the treatment of adults with relapsed or refractory chronic lymphocytic leukaemia (CLL) or refractory follicular lymphoma (FL).
The CHMP adopted a positive opinion for Ponvory (ponesimod) for the treatment of active relapsing forms of multiple sclerosis.
Drovelis and its duplicate Lydisilka, both containing the active substances estetrol and drospirenone, received positive opinions from the Committee for use as oral contraceptives.
The CHMP recommended granting a marketing authorisation for the hybrid medicine Efmody* (hydrocortisone) for the treatment of congenital adrenal hyperplasia (CAH) in patients aged 12 years and over. Hybrid applications rely in part on the results of pre-clinical tests and clinical trials of an already authorised reference product and in part on new data.
Six recommendations on extensions of therapeutic indication
The Committee recommended extensions of indication for Benlysta, Kaftrio, Kalydeco, Saxenda, Tecentriq and Xtandi.
Withdrawal of application
An application to extend the use of Brilique (ticagrelor) with aspirin (acetylsalicylic acid) to prevent problems caused by blood clots in adults with coronary artery disease and type 2 diabetes was withdrawn. A question-and-answer document on this withdrawal is available in the grid below.
COVID-19: Outcome of review on the use of regdanvimab
The CHMP gave a recommendation to define conditions of use for the monoclonal antibody regdanvimab (also known as CT-P59). This medicine is currently not authorised in the EU, but the recommendation provides a harmonised scientific opinion at EU level to support national decision making on the possible use of this antibody to treat confirmed COVID-19 in patients who do not require supplemental oxygen therapy and who are at high risk of progressing to severe COVID-19. See more details in the news announcement in the grid below.
Agenda and minutes
The agenda of the March 2021 CHMP meeting is published on EMA's website. Minutes of the February 2021 CHMP meeting will be published in the coming weeks.
CHMP statistics
Key figures from the March 2021 CHMP meeting are represented in the graphic below.
*This product was designated as an orphan medicine during its development. Orphan designations are reviewed by EMA's Committee for Orphan Medicinal Products (COMP) at the time of approval to determine whether the information available to date allows maintaining the medicine’s orphan status and granting the medicine ten years of market exclusivity.
Positive recommendations on new medicines
| Name of medicine | Copiktra |
| International non-proprietary name (INN) | duvelisib |
| Marketing-authorisation applicant | Verastem Europe GmbH |
| Therapeutic indication | Treatment of adult patients with relapsed or refractory chronic lymphocytic leukaemia (CLL) or refractory follicular lymphoma (FL) |
| More information | Copiktra: Pending EC decision |
| Name of medicine | Drovelis |
| International non-proprietary name (INN) | estetrol / drospirenone |
| Marketing-authorisation applicant | Gedeon Richter Plc. |
| Therapeutic indication | Oral contraception |
| More information | Drovelis: Pending EC decision |
| Name of medicine | Lydisilka |
| INN | estetrol / drospirenone |
| Marketing-authorisation applicant | Estetra SPRL |
| Therapeutic indication | Oral contraception |
| More information | Lydisilka: Pending EC decision |
| Name of medicine | Ponvory |
| INN | ponesimod |
| Marketing-authorisation applicant | Janssen-Cilag International N.V. |
| Therapeutic indication | Treatment of active relapsing forms of multiple sclerosis |
| More information | Ponvory: Pending EC decision |
Positive recommendation on new hybrid medicine
| Name of medicine | Efmody |
| INN | hydrocortisone |
| Marketing-authorisation applicant | Diurnal Europe BV |
| Therapeutic indication | Treatment of congenital adrenal hyperplasia (CAH) in patients aged 12 years and over |
| More information | Efmody: Pending EC decision |
Positive recommendations on extensions of indication
| Name of medicine | Benlysta |
| INN | belimumab |
| Marketing-authorisation holder | GlaxoSmithKline (Ireland) Limited |
| More information | Benlysta: Pending EC decision |
| Name of medicine | Kaftrio |
| INN | ivacaftor / tezacaftor / elexacaftor |
| Marketing-authorisation holder | Vertex Pharmaceuticals (Ireland) Limited |
| More information | Kaftrio: Pending EC decision |
| Name of medicine | Kalydeco |
| INN | ivacaftor |
| Marketing-authorisation holder | Vertex Pharmaceuticals (Ireland) Limited |
| More information | Kalydeco: Pending EC decision |
| Name of medicine | Saxenda |
| INN | liraglutide |
| Marketing-authorisation holder | Novo Nordisk A/S |
| More information | Saxenda:Pending EC decision |
| Name of medicine | Tecentriq |
| INN | atezolizumab |
| Marketing-authorisation holder | Roche Registration GmbH |
| More information | Tecentriq: Pending EC decision |
| Name of medicine | Xtandi |
| INN | enzalutamide |
| Marketing-authorisation holder | Astellas Pharma Europe B.V. |
| More information | Xtandi: Pending EC decision |
Withdrawal of extension of indication
| Name of medicine | Brillique |
| INN | ticagrelor |
| Marketing-authorisation holder | AstraZeneca AB |
| More information | Brillique: Withdrawn application |
Opinion on any scientific matter (Article 5(3))
| Name of medicine | Celltrion use of regdanvimab for the treatment of COVID-19 |
| INN | regdanvimab |
| More information | EMA issues advice on use of regdanvimab for treating COVID-19 |