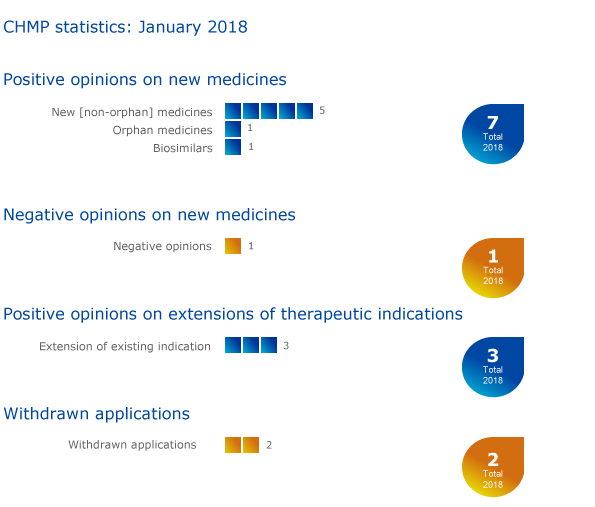
Seven medicines recommended for approval, including one orphan
The European Medicines Agency's (EMA) Committee for Medicinal Products for Human Use (CHMP) recommended seven medicines for approval, including one orphan medicine1, at its January 2018 meeting.
The CHMP recommended granting a marketing authorisation for Hemlibra (emicizumab), a first-in-class medicine to prevent bleeding episodes in patients with haemophilia A who have factor VIII inhibitors. This medicine was reviewed under EMA's accelerated assessment procedure, reserved for medicines of major public health interest. For more information, please see the press release in the grid below.
The CHMP recommended granting a marketing authorisation for Lamzede (velmanase alfa), a long-term enzyme replacement therapy in adults, adolescents and children with mild to moderate forms of alpha-mannosidosis. Because alpha-mannosidosis is a very rare disease, Lamzede was granted an orphan designation. For more information, please see the press release in the grid below.
The Committee recommended granting a marketing authorisation for Shingrix (recombinant, adjuvanted Herpes zoster vaccine), a vaccine for the prevention of herpes zoster and post-herpetic neuralgia in adults 50 years of age or older.
The CHMP gave positive opinions for three medicines to treat type 2 diabetes: Segluromet (ertugliflozin / metformin), Steglatro (ertugliflozin) and Steglujan (ertugliflozin / sitagliptin).
One biosimilar medicine was recommended for approval by the Committee: Semglee (insulin glargine), for the treatment of diabetes.
Re-adoption of opinion on new medicine
The CHMP confirmed its previous positive opinion and recommended the granting of a marketing authorisation for Lokelma (sodium zirconium cyclosilicate), for the treatment of hyperkalaemia.
Lokelma had received a positive opinion in February 2017. However, the European Commission suspended its decision-making following some concerns relating to good manufacturing practices (GMP) at the manufacturing site for the active substance and referred the matter back to the CHMP. A new inspection of the manufacturing site for Lokelma's active substance showed that the site is compliant with GMP. On the basis of the inspection findings, the Committee confirmed its previous opinion.
Negative opinion on new medicine
The CHMP adopted a negative opinion for EnCyzix (enclomifene). EnCyzix was expected to be used to treat hypogonadotrophic hypogonadism in men. For more information on this negative opinion, please see the questions-and-answers document in the grid below.
Start of re-examination of recommendation on new medicine
The applicant for Aplidin (plitidepsin) has requested a re-examination of the Committee's negative opinion for this medicine adopted at the December 2017 meeting. The CHMP will now re-examine this opinion and issue a final recommendation.
For more information on this negative opinion, please see the questions-and-answers document in the grid below.
Three recommendations on extensions of therapeutic indication
The Committee recommended extensions of indications for Hizentra, Relvar Ellipta and Revinty Ellipta.
Negative recommendation on extension of indication following re-examination
The applicant for Raxone (idebenone) requested a re-examination of the Committee's negative opinion for this medicine adopted at the September 2017 meeting. After considering the grounds for this request, the CHMP re-examined the opinion, and confirmed the refusal of the change to the marketing authorisation.
For more information on this negative opinion, please see the questions-and-answers document in the grid below.
Withdrawals of applications
Applications for initial marketing authorisations for Balimek (binimetinib) and Rotigotine Mylan (rotigotine) have been withdrawn. Balimek was intended to be used to treat melanoma (a type of skin cancer) that had spread or could not be removed by surgery. Rotigotine Mylan was intended to be used to treat Parkinson's disease and restless-leg syndrome.
An application to extend the use of Opdivo (nivolumab) to treat colorectal cancer has also been withdrawn.
Questions-and-answers documents on these withdrawals are available in the grid below.
Agenda and minutes
The agenda of the January 2018 meeting is published on EMA's website. Minutes of the December 2017 CHMP meeting will be published in the coming weeks.
CHMP statistics
Key figures from the January 2018 CHMP meeting are represented in the graphic below.
More information on all other outcomes of the CHMP January 2018 meeting is available in the grid below.
1As always at time of approval, this orphan designation will now be reviewed by EMA's Committee for Orphan Medicinal Products (COMP) to determine whether the information available to date allows maintaining the medicine's orphan status and granting the medicine ten years of market exclusivity.
Positive recommendations on new medicines
| Name of medicine | Hemlibra |
|---|---|
| International non-proprietary name (INN) | emicizumab |
| Marketing-authorisation applicant | Roche Registration Limited |
| Therapeutic indication | Prevention of bleeding episodes in patients with haemophilia A who have factor VIII inhibitors |
| More information | CHMP summary of positive opinion for Hemlibra
Press release: First-in-class medicine to prevent bleeding in haemophilia A patients with inhibitors |
| Name of medicine | Lamzede |
|---|---|
| INN | velmanase alfa |
| Marketing-authorisation applicant | Chiesi Farmaceutici S.p.A. |
| Therapeutic indication | Treatment of patients with non-neurological manifestations of mild to moderate alpha-mannosidosis |
| More information | CHMP summary of positive opinion for Lamzede
Press release: New enzyme replacement therapy to treat rare genetic disorder alpha-mannosidosis in children and adults |
| Name of medicine | Segluromet |
|---|---|
| INN | ertugliflozin / metformin |
| Marketing-authorisation applicant | Merck Sharp & Dohme Limited |
| Therapeutic indication | Treatment of type 2 diabetes |
| More information | CHMP summary of positive opinion for Segluromet |
| Name of medicine | Shingrix |
|---|---|
| Common name | herpes zoster vaccine (recombinant, adjuvanted) |
| Marketing-authorisation applicant | GlaxoSmithkline Biologicals SA |
| Therapeutic indication | Prevention of herpes zoster (HZ) and post-herpetic neuralgia (PHN), in adults 50 years of age or older |
| More information | CHMP summary of positive opinion for Shingrix |
| Name of medicine | Steglatro |
|---|---|
| INN | ertugliflozin |
| Marketing-authorisation applicant | Merck Sharp & Dohme Limited |
| Therapeutic indication | Treatment of type 2 diabetes |
| More information | CHMP summary of positive opinion for Steglatro |
| Name of medicine | Steglujan |
|---|---|
| INN | ertugliflozin / sitagliptin |
| Marketing-authorisation applicant | Merck Sharp & Dohme Limited |
| Therapeutic indication | Treatment of type 2 diabetes |
| More information | CHMP summary of positive opinion for Steglujan |
Positive recommendation on new biosimilar medicine
| Name of medicine | Semglee |
|---|---|
| INN | insulin glargine |
| Marketing-authorisation applicant | Mylan S.A.S |
| Therapeutic indication | Treatment of diabetes |
| More information | CHMP summary of positive opinion for Semglee |
Re-adoption of opinion for new medicine
| Name of medicine | Lokelma |
|---|---|
| INN | sodium zirconium cyclosilicate |
| Marketing-authorisation applicant | AstraZeneca AB |
| Therapeutic indication | Treatment of hyperkalaemia |
| More information | CHMP summary of positive opinion for Lokelma |
Negative recommendation for new medicine
| Name of medicine | EnCyzix |
|---|---|
| INN | enclomifene |
| Marketing-authorisation applicant | Renable Pharma Limited |
| Therapeutic indication | Treatment of hypogonadotropic hypogonadism in men |
| More information | Questions and answers on refusal of the marketing authorisation for EnCyzix (enclomifene) |
Start of re-examination of recommendation on new medicine
| Name of medicine | Aplidin |
|---|---|
| INN | plitidepsin |
| Marketing-authorisation applicant | Pharma Mar |
| Therapeutic indication | Treatment of multiple myeloma |
| More information |
Positive recommendations on extensions of indications
| Name of medicine | Hizentra |
|---|---|
| INN | human normal immunoglobulin |
| Marketing-authorisation holder | CSL Behring GmbH |
| More information | CHMP post-authorisation summary of positive opinion for Hizentra (II-0087) |
| Name of medicine | Relvar Ellipta |
|---|---|
| INN | fluticasone furoate / vilanterol |
| Marketing-authorisation holder | Glaxo Group Ltd |
| More information | CHMP post-authorisation summary of positive opinion for Relvar Ellipta (WS-1208) |
| Name of medicine | Revinty Ellipta |
|---|---|
| INN | fluticasone furoate / vilanterol |
| Marketing-authorisation holder | Glaxo Group Ltd |
| More information | CHMP post-authorisation summary of positive opinion for Revinty Ellipta |
Negative recommendation on extension of indication following re-examination
| Name of medicine | Raxone |
|---|---|
| INN | idebenone |
| Marketing-authorisation holder | Santhera Pharmaceuticals (Deutschland) GmbH |
| More information | Questions and answers on the refusal of a change to the marketing authorisation for Raxone (idebenone) |
Withdrawals of initial marketing autorisation applications
| Name of medicine | Balimek |
|---|---|
| INN | binimetinib |
| Marketing-authorisation applicant | Pierre Fabre Médicament |
| More information | Questions and answers on the withdrawal of the marketing authorisation application for Balimek (binimetinib) |
| Name of medicine | Rotigotine Mylan |
|---|---|
| INN | rotigotine |
| Marketing-authorisation applicant | Mylan S.A.S |
| More information | Questions and answers on withdrawal of the marketing authorisation application for Rotigotine Mylan (rotigotine) |
Withdrawal of extension of indication application
| Name of medicine | Opdivo |
|---|---|
| INN | nivolumab |
| Marketing-authorisation holder | Bristol-Myers Squibb Pharma EEIG |
| More information | Questions and answers on the withdrawal of the application for a change to the marketing authorisation for Opdivo (nivolumab) |
Other updates
| Recommendations on eligibility to PRIME scheme - Adopted at the CHMP meeting of 22-25 January 2018 |
| Scientific advice and protocol assistance adopted during the CHMP meeting 22 – 25 January 2018 |
| Overview of (invented) names reviewed in December 2017 by the Name Review Group (NRG) |