Six medicines, including two orphan medicines, recommended for approval
The European Medicines Agency's Committee for Medicinal Products for Human Use (CHMP) recommended six new medicines for marketing authorisation at its February 2016 meeting.
The CHMP recommended granting marketing authorisations for two medicines for the prevention and treatment of bleeding in patients with haemophilia B, Alprolix (eftrenonacog alfa) and Idelvion (albutrepenonacog alfa). Both these medicines have an orphan designation.
Lonsurf (trifluridine / tipiracil) was recommended by the CHMP for the treatment of metastatic colorectal cancer.
Descovy (emtricitabine / tenofovir alafenamide) received a positive opinion for the treatment of HIV infection.
The CHMP recommended granting a marketing authorisation for Taltz (ixekizumab) for the treatment of plaque psoriasis.
The generic medicine Palonosetron Hospira (palonosetron) received a positive recommendation from the Committee for the prevention of nausea and vomiting associated with chemotherapy.
Seven recommendations on extensions of therapeutic indications
The Committee recommended extensions of indications for Giotrif, Humira, Ruconest, TachoSil, Zydelig and two extensions of indications for Opdivo. For more information on the extensions of indications for Opdivo, please see the press release in the grid below.
Start of review: medicines containing dienogest and ethinylestradiol
The CHMP started a review of medicines containing dienogest 2 mg and ethinylestradiol 0.03 mg when used for acne. These products are available in several countries in the European Union (EU) as oral contraceptives and for the treatment of moderate acne in women. For more information, please refer to the start of referral document in the grid below.
Outcome of review on SGLT2 inhibitors: recommendations to minimise risk of diabetic ketoacidosis
The CHMP confirmed recommendations from the Pharmacovigilance Risk Assessment Committee (PRAC) to minimise the risk of diabetic ketoacidosis in patients taking SGLT2 inhibitors (a class of type 2 diabetes medicines). For more information, please see the public health communication in the grid below.
CHMP confirms recommendations to minimise risk of the brain infection PML with Tysabri
EMA's scientific review of the known risk of progressive multifocal leukoencephalopathy (PML) with the multiple sclerosis medicine Tysabri (natalizumab) is now completed, with the CHMP confirming the PRAC recommendations aimed at minimising this risk. For more information, please see the public health communication in the grid below.
PRIME and early access tools
The CHMP adopted the final document on PRIME, a new scheme to support the development of medicines addressing unmet medical needs, as well as revised guidelines on the implementation of accelerated assessment and conditional marketing authorisation. These documents will be published on the EMA website in early March.
Agenda and minutes
The agenda of the February 2016 meeting is published on EMA's website. Minutes of the January 2016 CHMP meeting will be published next week.
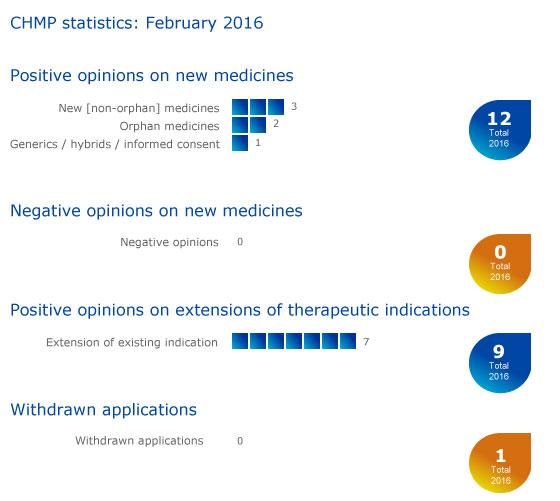
CHMP statistics
Key figures from the February 2016 CHMP meeting are represented in the graphic below.
More information on this, and all other outcomes of the CHMP's February 2016 meeting, is available in the grid below.
CHMP statistics: February 2016
Positive recommendations on new medicines
| Name of medicine | Alprolix |
|---|---|
| INN | eftrenonacog alfa |
| Marketing-authorisation applicant | Biogen Idec Ltd |
| Therapeutic indication | Treatment and prophylaxis of bleeding in patients with haemophilia B |
| More information | CHMP summary of opinion for Alprolix |
| Name of medicine | Descovy |
|---|---|
| INN | emtricitabine / tenofovir alafenamide |
| Marketing-authorisation applicant | Gilead Sciences International Ltd |
| Therapeutic indication | Treatment of HIV infection |
| More information | CHMP summary of positive opinion for Descovy |
| Name of medicine | Idelvion |
|---|---|
| INN | albutrepenonacog alfa |
| Marketing-authorisation applicant | CSL Behring GmbH |
| Therapeutic indication | Treatment and prophylaxis of bleeding in patients with haemophilia B |
| More information | CHMP summary of positive opinion for Idelvion |
| Name of medicine | Lonsurf |
|---|---|
| INN | trifluridine / tipiracil |
| Marketing-authorisation applicant | Les Laboratoires Servier |
| Therapeutic indication | Treatment of metastatic colorectal cancer |
| More information | CHMP summary of positive opinion for Lonsurf |
| Name of medicine | Taltz |
|---|---|
| INN | ixekizumab |
| Marketing-authorisation applicant | Eli Lilly Nederland B.V. |
| Therapeutic indication | Treatment of plaque psoriasis |
| More information | CHMP summary of positive opinion for Taltz |
Positive recommendation on new generic medicine
| Name of medicine | Palonosetron Hospira |
|---|---|
| INN | palonosetron |
| Marketing-authorisation applicant | Hospira UK Limited |
| Therapeutic indication | Prevention of nausea and vomiting associated with cancer chemotherapy |
| More information | CHMP summary of opinion for Palonosetron Hospira |
Positive recommendations on extensions of therapeutic indications
| Name of medicine | Giotrif |
|---|---|
| INN | afatinib |
| Marketing-authorisation holder | Boehringer Ingelheim International GmbH |
| More information | CHMP post-authorisation summary of positive opinion for Giotrif |
| Name of medicine | Humira |
|---|---|
| INN | adalimumab |
| Marketing-authorisation holder | AbbVie Ltd |
| More information | CHMP post-authorisation summary of positive opinion for Humira |
| Name of medicine | Opdivo |
|---|---|
| INN | nivolumab |
| Marketing-authorisation holder | Bristol-Myers Squibb Pharma EEIG |
| More information | CHMP post-authorisation summary of positive opinion for Opdivo (II/0002)
Press release: New treatment for advanced form of kidney cancer |
| Name of medicine | Ruconest |
|---|---|
| INN | conestat alfa |
| Marketing-authorisation holder | Pharming Group N.V |
| More information | CHMP post-authorisation summary of positive opinion for Ruconest |
| Name of medicine | TachoSil |
|---|---|
| INN | human thrombin / human fibrinogen |
| Marketing-authorisation holder | Takeda Austria GmbH |
| More information | CHMP post-authorisation summary of positive opinion for TachoSil |
| Name of medicine | Zydelig |
|---|---|
| INN | idelalisib |
| Marketing-authorisation holder | Gilead Sciences International Ltd |
| More information | CHMP post-authorisation summary of positive opinion for Zydelig |
Recommendation for new contraindication
| Name of medicine | Telzir |
|---|---|
| INN | fosamprenavir |
| Marketing-authorisation holder | ViiV Healthcare UK Limited |
| More information | CHMP post-authorisation summary of positive opinion for Telzir |
Start of referral
| Name of medicine | Dienogest / Ethinylestradiol containing medicinal products indicated in acne |
|---|---|
| INN | dienogest / ethinylestradiol |
| More information | Start of review of medicines containing dienogest 2 mg and ethinylestradiol 0.03 mg for acne |
Public health recommendations
| Name of medicine | Tysabri |
|---|---|
| INN | natalizumab |
| Marketing-authorisation holder | Biogen Idec Ltd |
| More information | EMA confirms recommendations to minimise risk of brain infection PML with Tysabri |
| Name of medicine | SGLT2 inhibitors |
|---|---|
| INN | canagliflozin, dapagliflozin, empagliflozin |
| Marketing-authorisation holder | AstraZeneca AB (Forxiga, Xigduo), Boehringer Ingelheim International GmbH (Jardiance, Synjardy), Janssen-Cilag International N.V. (Invokana, Vokanamet) |
| More information | EMA confirms recommendations to minimise ketoacidosis risk with SGLT2 inhibitors for diabetes |
Outcome of harmonisation procedure
| Name of medicine | Cymevene |
|---|---|
| INN | ganciclovir |
| Marketing-authorisation holder | F. Hoffmann-La Roche |
| More information | Questions and answers on Cymevene and associated names (ganciclovir, 500 mg powder for concentrate for solution for infusion, intravenous |