Six medicines recommended for approval, including three orphans
The European Medicines Agency's Committee for Medicinal Products for Human Use (CHMP) recommended six medicines for approval at its March 2017 meeting.
The CHMP recommended granting a marketing authorisation under exceptional circumstances for Dinutuximab beta Apeiron (dinutuximab beta) for the treatment of high-risk neuroblastoma. This medicine has an orphan designation. Authorisation under exceptional circumstances allows patients access to medicines that cannot be approved under a standard authorisation as comprehensive data cannot be obtained, either because there are only very few patients with the disease, the collection of complete information on the efficacy and safety of the medicine would be unethical, or there are gaps in the scientific knowledge. These medicines are subject to specific post-authorisation obligations and monitoring.
The Committee granted a positive opinion for Refixia (nonacog beta pegol) for the treatment and prophylaxis of bleeding in patients 12 years and above with haemophilia B (congenital factor IX deficiency). Refixia has an orphan designation.
The CHMP granted a positive opinion for Elmiron (pentosan polysulfate sodium) for the treatment of bladder pain syndrome characterised by either glomerulations or Hunner's lesions (tiny bleeds or distinctive lesions on the bladder wall). Elmiron has an orphan designation.
Trumenba, a meningococcal group B vaccine (recombinant, adsorbed) to prevent invasive meningococcal disease caused by meningococcal serogroup B bacteria, received a positive opinion from the Committee.
A diagnostic agent, Axumin (fluciclovine (18F)), was recommended for marketing authorisation by the Committee for the detection of recurrence of prostate cancer with positron emission tomography (PET) imaging.
A generic medicine, Ivabradine Accord (ivabradine), received a positive opinion from the Committee for the treatment of angina pectoris and chronic heart failure.
Three recommendations on extensions of therapeutic indications
The Committee recommended extensions of indications for Keytruda, Opdivo and Zebinix.
Withdrawals of applications
Applications for initial marketing authorisations for Blectifor (caffeine citrate) and Enpaxiq (pacritinib) have been withdrawn.
An application to extend the indication of Translarna (ataluren) to treat cystic fibrosis has also been withdrawn.
Questions-and-answers documents on these withdrawals are available in the grid below.
CHMP recommends suspension of medicines due to unreliable studies from Micro Therapeutic Research Labs
The CHMP recommended suspending a number of nationally approved medicines for which bioequivalence studies were conducted by Micro Therapeutic Research Labs at two sites in India. The suspensions can be lifted once alternative data establishing bioequivalence are provided. More information, including the list of medicines recommended for suspension, is available in the public health communication in the grid below.
Agenda and minutes
The agenda of the March 2017 meeting is published on EMA's website. Minutes of the February 2017 CHMP meeting will be published next week.
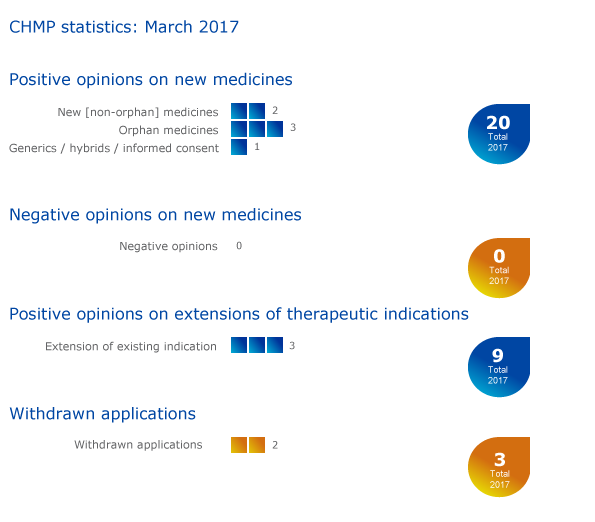
CHMP statistics
Key figures from the March 2017 CHMP meeting are represented in the graphic below.
More information on all other outcomes of the CHMP's March 2017 meeting is available in the grid below.
Positive recommendations on new medicines
| Name of medicine | Axumin |
|---|---|
| INN | fluciclovine (18F) |
| Marketing authorisation applicant | Blue Earth Diagnostics Ltd |
| Therapeutic indication | Diagnostic agent for the detection of recurrence of prostate cancer with positron emission tomography (PET) imaging |
| More information | CHMP summary of positive opinion for Axumin |
| Name of medicine | Dintuximab beta Apeiron |
|---|---|
| INN | dinutuximab beta |
| Marketing authorisation applicant | Apeiron Biologics AG |
| Therapeutic indication | Treatment of high-risk neuroblastoma |
| More information | CHMP summary of positive opinion for Dinutuximab beta Apeiron |
| Name of medicine | Elmiron |
|---|---|
| INN | pentosan polysulfate sodium |
| Marketing authorisation applicant | bene-Arzneimittel GmbH |
| Therapeutic indication | Treatment of bladder pain syndrome characterised by either glomerulations or Hunner's lesions |
| More information | CHMP summary of positive opinion for Elmiron |
| Name of medicine | Refixia |
|---|---|
| INN | nonacog beta pegol |
| Marketing authorisation applicant | Novo Nordisk A/S |
| Therapeutic indication | Treatment of haemophilia B |
| More information | CHMP summary of positive opinion for Refixia |
| Name of medicine | Trumenba |
|---|---|
| Common name | meningococcal group B vaccine (recombinant, adsorbed) |
| Marketing authorisation applicant | Pfizer Limited |
| Therapeutic indication | Prophylaxis against invasive meningococcal disease caused by meningococcal serogroup B bacteria |
| More information | CHMP summary of positive opinion for Trumenba |
Positive recommendation on new generic medicine
| Name of medicine | Ivabradine Accord |
|---|---|
| INN | ivabradine |
| Marketing authorisation applicant | Accord Healthcare Ltd |
| Therapeutic indication | Treatment of angina pectoris and chronic heart failure |
| More information | CHMP summary of positive opinion for Ivabradine Accord |
Positive recommendations on extensions of therapeutic indications
| Name of medicine | Keytruda |
|---|---|
| INN | pembrolizumab |
| Marketing authorisation holder | Merck Sharp & Dohme Limited |
| More information | CHMP post-authorisation summary of positive opinion for Keytruda (II-0014) |
| Name of medicine | Opdivo |
|---|---|
| INN | nivolumab |
| Marketing authorisation holder | Bristol-Myers Squibb Pharma EEIG |
| More information | CHMP post-authorisation summary of positive opinion for Opdivo |
| Name of medicine | Zebinix |
|---|---|
| INN | eslicarbazepine acetate |
| Marketing authorisation holder | Bial - Portela & Cª, S.A. |
| More information | CHMP post-authorisation summary of positive opinion for Zebinix |
Public health recommendation
| Name of medicine | Micro Therapeutics Research Labs, India |
|---|---|
| More information | EMA recommends suspension of medicines due to unreliable studies from Micro Therapeutic Research Labs |
Withdrawals of applications
| Name of medicine | Blectifor |
|---|---|
| INN | caffeine citrate |
| Marketing authorisation applicant | Viridian Pharma Ltd |
| More information | Questions and answers on the withdrawal of the marketing authorisation application for Blectifor (caffeine citrate) |
| Name of medicine | Enpaxiq |
|---|---|
| INN | pacritinib |
| Marketing authorisation applicant | CTI Life Sciences Limited |
| More information | Questions and answers on the withdrawal of the marketing authorisation application for Enpaxiq (pacritinib) |
| Name of medicine | Translarna |
|---|---|
| INN | ataluren |
| Marketing authorisation holder | PTC Therapeutics International Limited |
| More information | Questions and answers on the withdrawal of the application for a change to the marketing authorisation for Translarna (ataluren) |
Other updates