Two new medicines recommended for approval
EMA’s human medicines committee (CHMP) recommended two medicines for approval at its June 2023 meeting.
The committee recommended granting a marketing authorisation for Aquipta (atogepant), intended for the prophylaxis of migraine in adults who have at least four migraine days per month. It is estimated that approximately 15% of the EU population suffers from migraine, a type of headache characterised by recurrent attacks of moderate to severe throbbing and pulsating pain on one side of the head.
Jesduvroq (daprodustat) received a positive opinion from the CHMP for the treatment of adult patients with anaemia associated with chronic kidney disease, a condition in which the kidneys are damaged and cannot filter the blood as well as they should.
Negative opinion for a new medicine
The CHMP recommended the refusal of a marketing authorisation for Albrioza* (sodium phenylbutyrate/ursodoxicoltaurine) for the treatment of amyotrophic lateral sclerosis, a rare neurological disease affecting nerve cells in the brain and spinal cord that control voluntary muscle movement. For more information on this negative opinion, see the question-and-answer document in the grid below.
Withdrawals of applications
Applications for the biosimilar medicines Dyrupeg and Zefylti were withdrawn. Both of these medicines were intended for the treatment of neutropenia, a condition that affects the immune system, but they were developed as biosimilars of different active substances.
Question-and-answer documents on the withdrawals are available in the grid below.
Recommendations on extensions of therapeutic indication for eight medicines
The committee recommended eight extensions of indication for medicines that are already authorised in the European Union (EU): Comirnaty, Imjudo, Jardiance, Lonsurf, Mircera, Refixia, Soliris* and Trodelvy.
Agenda and minutes
The agenda of the June 2023 CHMP meeting is published on EMA's website. Minutes of the May 2023 CHMP meeting will be published in the coming weeks.
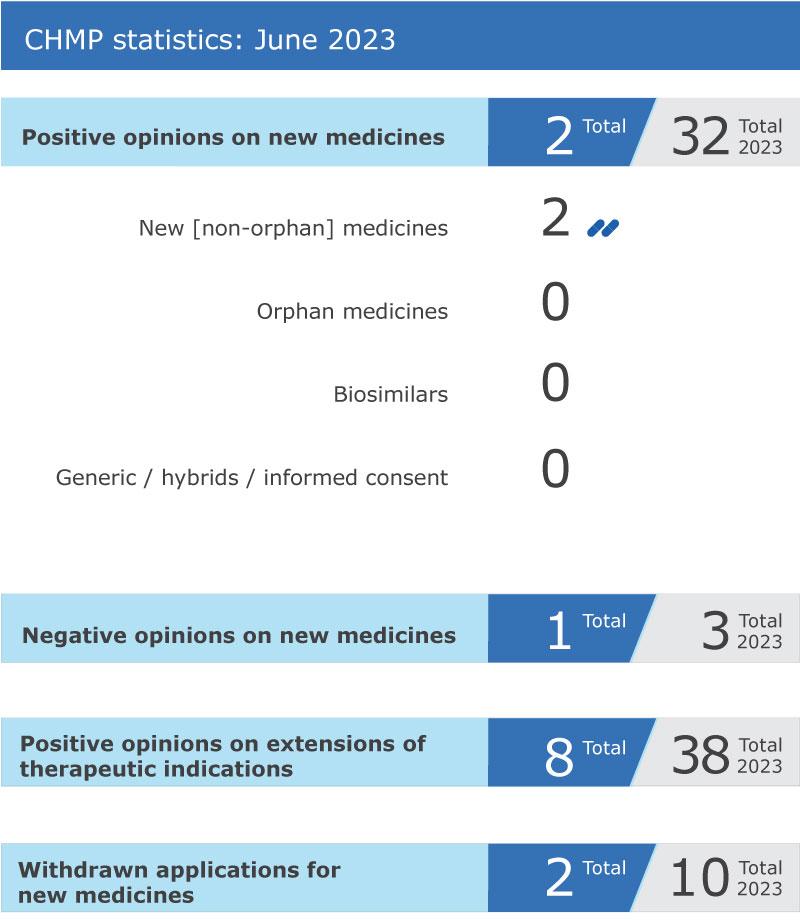
CHMP statistics
Key figures from the June 2023 CHMP meeting are represented in the graphic below.
*This product was designated as an orphan medicine during its development. Orphan designations are reviewed by EMA's Committee for Orphan Medicinal Products (COMP) at the time of approval to determine whether the information available to date allows maintaining the medicine’s orphan status and granting the medicine ten years of market exclusivity.
Positive recommendations on new medicines
| Name of medicine | Aquipta |
|---|---|
| International non-proprietary name (INN) | atogepant |
| Marketing-authorisation applicant | AbbVie Deutschland GmbH & Co. KG |
| Therapeutic indication | Prophylaxis of migraine in adults who have at least 4 migraine days per month |
| More information | Aquipta: Pending EC decision |
| Name of medicine | Jesduvroq |
|---|---|
| INN | daprodustat |
| Marketing-authorisation applicant | Glaxosmithkline Trading Services Limited |
| Therapeutic indication | Treatment of anaemia associated with chronic kidney disease (CKD) in adults |
| More information | Jesduvroq: Pending EC decision |
Negative recommendations on new medicines
| Name of medicine | Albrioza |
|---|---|
| INN | sodium phenylbutyrate / ursodoxicoltaurine |
| Marketing-authorisation applicant | Amylyx Pharmaceuticals EMEA B.V. |
| Therapeutic indication | Treatment of amyotrophic lateral sclerosis (ALS) |
| More information | Albrioza: Pending EC decision |
Positive recommendations on extensions of indications
| Name of medicine | Comirnaty |
|---|---|
| INN | tozinameran / riltozinameran and tozinameran / famtozinameran and tozinameran / COVID-19 mRNA Vaccine (nucleoside modified) |
| Marketing-authorisation holder | BioNTech Manufacturing GmbH |
| More information | Comirnaty: Pending EC decision |
| Name of medicine | Imjudo |
|---|---|
| INN | tremelimumab |
| Marketing-authorisation holder | AstraZeneca AB |
| More information | Imjudo: Pending EC decision |
| Name of medicine | Jardiance |
|---|---|
| INN | empagliflozin |
| Marketing-authorisation holder | Boehringer Ingelheim International GmbH |
| More information | Jardiance: Pending EC decision |
| Name of medicine | Lonsurf |
|---|---|
| INN | trifluridine / tipiracil |
| Marketing-authorisation holder | Les Laboratoires Servier |
| More information | Lonsurf: Pending EC decision |
| Name of medicine | Mircera |
|---|---|
| INN | methoxy polyethylene glycol-epoetin beta |
| Marketing-authorisation holder | Roche Registration GmbH |
| More information | Mircera: Pending EC decision |
| Name of medicine | Refixia |
|---|---|
| INN | nonacog beta pegol |
| Marketing-authorisation holder | Novo Nordisk A/S |
| More information | Refixia: Pending EC decision |
| Name of medicine | Soliris |
|---|---|
| INN | eculizumab |
| Marketing-authorisation holder | Alexion Europe SAS |
| More information | Soliris: Pending EC decision |
| Name of medicine | Trodelvy |
|---|---|
| INN | sacituzumab govitecan |
| Marketing-authorisation holder | Gilead Sciences Ireland UC |
| More information | Trodelvy: Pending EC decision |
| Name of medicine | Dyrupeg |
|---|---|
| INN | pegfilgrastim |
| Marketing-authorisation applicant | CuraTeQ Biologics s.r.o |
| More information | Questions and answers on Dyrupeg |
| Name of medicine | Zefylti |
|---|---|
| INN | filgrastim |
| Marketing-authorisation applicant | CuraTeQ Biologics s.r.o |
| More information | Questions and answsers on Zefylti |