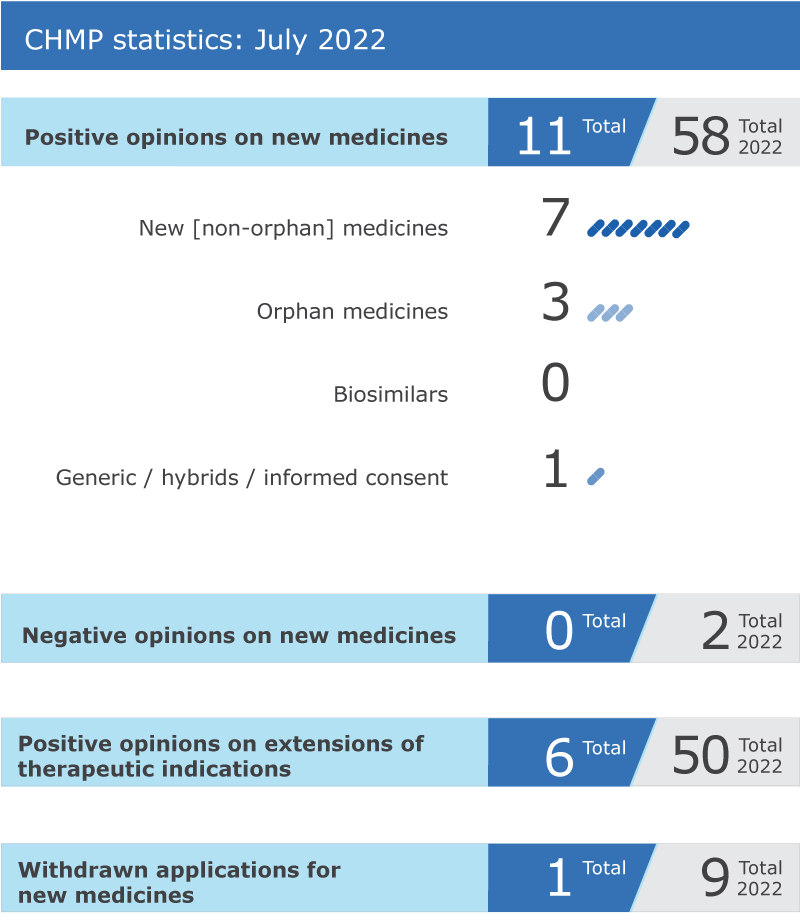
11 new medicines recommended for approval
EMA’s human medicines committee (CHMP) recommended 11 medicines for approval at its July 2022 meeting.
The CHMP recommended granting a marketing authorisation for Amvuttra* (vutrisiran) for the treatment of adults with hereditary transthyretin-mediated amyloidosis, a rare life-threatening disease that damages multiple nerves across the body.
The committee adopted a positive opinion for Celdoxome pegylated liposomal (doxorubicin hydrochloride) for the treatment of metastatic breast cancer, advanced ovarian cancer, progressive multiple myeloma and Kaposi's sarcoma, a type of cancer that affects people with AIDS.
Illuzyce (lutetium (177lu) chloride), a radiopharmaceutical precursor, received a positive opinion from the CHMP. Illuzyce is not intended for direct use in patients and must be used only for the radiolabelling of carrier medicines that have been specifically developed and authorised for radiolabelling with lutetium chloride.
The CHMP recommended granting a marketing authorisation for Lupkynis (voclosporin) for the treatment of lupus nephritis, an inflammation of the kidney caused by lupus. Lupus is an auto immune disease in which the body's immune system attacks healthy tissues.
The committee adopted a positive opinion for Mounjaro (tirzepatide) for the treatment of adults with type 2 diabetes mellitus. Around 30 million people suffer from diabetes in the European Union (EU).
The CHMP gave a positive opinion under exceptional circumstances for Nulibry* (fosdenopterin) for the treatment of molybdenum cofactor deficiency type A. This is an ultra-rare condition that appears shortly after birth and leads to brain injury and death.
Opdualag (relatlimab / nivolumab), intended for the treatment of melanoma, a type of skin cancer, that has spread to other parts of the body and cannot be removed by surgery, received a positive opinion from the CHMP.
The committee recommended granting a conditional marketing authorisation for Tecvayli* (teclistamab) for the treatment of adults with relapsed and refractory multiple myeloma, who have received at least three prior therapies. Multiple myeloma is a rare cancer of the bone marrow that affects plasma cells, a type of white blood cell that produces antibodies. Tecvayli was supported through EMA's PRIority MEdicines (PRIME) scheme, which provides early and enhanced scientific and regulatory support for medicines that have a particular potential to address patients' unmet medical needs. The CHMP reviewed the application for marketing authorisation under an accelerated timetable to enable faster patient access to this medicine. See more information in the news announcement in the grid below.
The CHMP gave a positive opinion for Tezspire (Tezepelumab), intended as an add-on treatment in adult and adolescent patients with severe asthma. Asthma is a chronic condition that affects around 6% of the EU population.
Vabysmo (faricimab), intended for the treatment of adults with neovascular age-related macular degeneration and visual impairment due to diabetic macular oedema, received a positive opinion from the committee.
Hybrid medicine Thalidomide Lipomed (thalidomide) received a positive opinion for the treatment of multiple myeloma. Hybrid medicines rely in part on the results of pre-clinical tests and clinical trials of an already authorised reference product and in part on new data.
Recommendations on extensions of therapeutic indication for six medicines
The committee recommended six extensions of indication for medicines that are already authorised in the EU: Genvoya, Imcivree, Retsevmo, Tecartus and Ultomiris.
The CHMP has also recommended an extension of the use of the smallpox vaccine Imvanex (live modified vaccinia virus Ankara) to include protecting adults from monkeypox and disease caused by vaccinia virus. More information on this extension of indication is available in the news announcement in the grid below.
Outcome of referral
The committee has recommended that Rubraca (rucaparib camsylate) should no longer be used as third-line treatment for cancers of the ovary, fallopian tubes or peritoneum with a BRCA mutation in patients whose cancer has come back after at least two platinum-based chemotherapies and who cannot have further platinum-based therapy. The review was carried out under Article 20 of Regulation (EC) No 726/2004, which is triggered for centrally authorised medicines in case of quality, safety and efficacy issues. For more information, see the public health communication in the grid below.
Withdrawals of applications
The marketing authorisation holder for Imcivree (setmelanotide) withdrew its application to extend the use of this medicine to treat obesity and control hunger associated with genetically confirmed Alström syndrome, a rare genetic disease that causes a variety of problems in several organs across the body. A question-and-answer document on the withdrawal is available in the grid below.
The application for a marketing authorisation for Parsaclisib Incyte Biosciences Distribution B.V. (parsaclisib) was withdrawn by the applicant. The medicine was intended for the treatment of marginal zone lymphoma, a cancer of the white blood cells. A question-and-answer document on the withdrawal is available in the grid below.
Covid-19 update
The CHMP recommended extending the use of COVID-19 vaccine Spikevax (elasomeran / COVID-19 mRNA vaccine (nucleoside-modified)) as a booster in adolescents from 12 to 17 years of age. The committee also endorsed updating the product information to state that stability has been demonstrated for 12 months when Spikevax is stored under certain conditions. The CHMP also recommended approving a new manufacturing site for the finished product in Madrid, Spain.
The committee recommended granting a full marketing authorisation to Veklury (remdesivir), an antiviral medicine used to treat COVID-19, following the submission of data by the marketing authorisation holder to fulfil the last outstanding specific obligation. Veklury is used in adults and adolescents with pneumonia requiring supplemental oxygen. The medicine can also be used in adults who do not require supplemental oxygen and who are at increased risk of developing severe COVID-19. Veklury received a conditional marketing authorisation in July 2020, which was last renewed in March 2022. It is the first COVID-19 treatment for which the CHMP recommended to receive a full marketing authorisation.
Agenda and minutes
The agenda of the July 2022 CHMP meeting is published on EMA's website. Minutes of the June 2022 CHMP meeting will be published in the coming weeks.
CHMP statistics
Key figures from the July 2022 CHMP meeting are represented in the graphic below.
*This product was designated as an orphan medicine during its development. Orphan designations are reviewed by EMA's Committee for Orphan Medicinal Products (COMP) at the time of approval to determine whether the information available to date allows maintaining the medicine’s orphan status and granting the medicine ten years of market exclusivity.
Positive recommendations on new medicines
| Name of medicine | Amvuttra |
| International non-proprietary name (INN) | vutrisiran |
| Marketing-authorisation applicant | Alnylam Netherlands B.V. |
| Therapeutic indication | Treatment of hereditary transthyretin-mediated (hATTR) amyloidosis |
| More information | Amvuttra: Pending EC decision |
| Name of medicine | Celdoxome pegylated liposomal |
| INN | doxorubicin hydrochloride |
| Marketing-authorisation applicant | YES Pharmaceutical Development Services GmbH |
| Therapeutic indication | Treatment of metastatic breast cancer, advanced ovarian cancer, progressive multiple myeloma and AIDS-related Kaposi's sarcoma |
| More information | Celdoxome pegylated liposomal: Pending EC decision |
| Name of medicine | Illuzyce |
| INN | lutetium (177lu) chloride |
| Marketing-authorisation applicant | Billev Pharma Aps |
| Therapeutic indication | Radiolabelling of carrier medicines that have been specifically developed and authorised for radiolabelling with lutetium (177Lu) chloride |
| More information | Illuzyce: Pending EC decision |
| Name of medicine | Lupkynis |
| INN | voclosporin |
| Marketing-authorisation applicant | Otsuka Pharmaceutical Netherlands B.V. |
| Therapeutic indication | Treatment of lupus nephritis |
| More information | Lupkynis: Pending EC decision |
| Name of medicine | Mounjaro |
| INN | tirzepatide |
| Marketing-authorisation applicant | Eli Lilly Nederland B.V. |
| Therapeutic indication | Treatment of adults with type 2 diabetes mellitus |
| More information | Mounjaro: Pending EC decision |
| Name of medicine | Nulibry |
| INN | fosdenopterin |
| Marketing-authorisation applicant | Comharsa Life Sciences Ltd |
| Therapeutic indication | Treatment of molybdenum cofactor deficiency (MoCD) Type A |
| More information | Nulibry: Pending EC decision |
| Name of medicine | Opdualag |
| INN | relatlimab / nivolumab |
| Marketing-authorisation applicant | Bristol-Myers Squibb Pharma EEIG |
| Therapeutic indication | Treatment of melanoma |
| More information | Opdualag: Pending EC decision |
| Name of medicine | Tecvayli |
| INN | teclistamab |
| Marketing-authorisation applicant | Janssen-Cilag International N.V. |
| Therapeutic indication | Treatment of relapsed or refractory multiple myeloma |
| More information | News:New medicine for multiple myeloma patients with limited treatment options |
| Name of medicine | Tezspire |
| INN | tezepelumab |
| Marketing-authorisation holder | AstraZeneca AB |
| Therapeutic indication | Add-on treatment in adult and adolescent patients with severe asthma |
| More information | Tezspire: Pending EC decision |
| Name of medicine | Vabysmo |
| INN | faricimab |
| Marketing-authorisation applicant | Roche Registration GmbH |
| Therapeutic indication | Treatment of neovascular (wet) age-related macular degeneration (nAMD) and visual impairment due to diabetic macular oedema (DME) |
| More information | Vabysmo: Pending EC decision |
Positive recommendation on new hybrid medicine
| Name of medicine | Thalidomide Lipomed |
| INN | thalidomide |
| Marketing-authorisation applicant | Lipomed GmbH |
| Therapeutic indication | Treatment of multiple myeloma |
| More information | Thalidomide Lipomed: Pending EC decision |
| Name of medicine | Parsaclisib Incyte Biosciences Distribution B.V. |
| INN | paraclisib |
| Marketing-authorisation applicant | Incyte Biosciences Distribution B.V. |
| Therapeutic indication | Treatment of marginal zone lymphoma |
| More information | Parsaclisib Incyte Biosciences Distribution B.V.: Withdrawal of the marketing authorisation application |
Positive recommendations on extensions of indications
| Name of medicine | Genvoya |
| INN | elvitegravir / cobicistat / emtricitabine / tenofovir alafenamide |
| Marketing-authorisation holder | Gilead Sciences Ireland UC |
| More information | Genvoya: Pending EC decision |
| Name of medicine | Imcivree |
| INN | setmelanotide |
| Marketing-authorisation holder | Rhythm Pharmaceuticals Netherlands B.V. |
| More information | Imcivree: Pending EC decision |
| Name of medicine | Imvanex |
| Common name | smallpox and monkeypox vaccine (Live Modified Vaccinia Virus Ankara) |
| Marketing-authorisation holder | Bavarian Nordic A/S |
| More information | News:EMA recommends approval of Imvanex for the prevention of monkeypox disease |
| Name of medicine | Retsevmo |
| INN | selpercatinib |
| Marketing-authorisation holder | Eli Lilly Nederland B.V. |
| More information | Retsevmo: Pending EC decision |
| Name of medicine | Tecartus |
| INN | brexucabtagene autoleucel |
| Marketing-authorisation holder | Kite Pharma EU B.V. |
| More information | Tecartus: Pending EC decision |
| Name of medicine | Ultomiris |
| INN | ravulizumab |
| Marketing-authorisation holder | Alexion Europe SAS |
| More information | Ultomiris: Pending EC decision |
| Name of medicine | Imcivree |
| INN | setmelanotide |
| Marketing-authorisation holder | Rhythm Pharmaceuticals Netherlands B.V. |
| More information | Imcivree: Withdrawn application |
Conclusion of referral
| Name of medicine | Rubraca |
| INN | rucaparib |
| Marketing-authorisation holder | Clovis Oncology Ireland Limited |
| More information | News:EMA recommends restricting use of cancer medicine Rubraca |