Seven medicines recommended for approval, including an advanced therapy
The European Medicines Agency's Committee for Medicinal Products for Human Use (CHMP) recommended seven medicines for approval at its December 2017 meeting, including two orphan medicines1, one of which is also an advanced therapy medicinal product (ATMP).
The CHMP recommended granting a marketing authorisation for the ATMP Alofisel (darvadstrocel), for the treatment of complex perianal fistulas in patients with Crohn's disease. Alofisel has an orphan designation. For more information, please see the press release in the grid below.
The Committee recommended granting a paediatric-use marketing authorisation (PUMA) for Alkindi (hydrocortisone), for the treatment of primary adrenal insufficiency, a rare hormonal disorder in infants, children and adolescents. PUMAs can be granted for medicines which are already authorised but no longer under patent protection, and have been developed specifically for children. For more information, please see the press release in the grid below.
The CHMP recommended granting a conditional marketing authorisation for Crysvita (burosumab), for the treatment of X-linked hypophosphataemia with radiographic evidence of bone disease in children and adolescents with growing skeletons. Crysvita has an orphan designation. For more information, please see the press release in the grid below.
Ozempic (semaglutide) received a positive opinion for the treatment of type 2 diabetes.
One biosimilar medicine was recommended for approval by the Committee: Herzuma (trastuzumab), for the treatment of breast and gastric cancer.
Two generic medicines received a positive opinion from the CHMP: Anagrelide Mylan (anagrelide), for the reduction of elevated platelet counts in at risk essential thrombocythaemia patients; and Efavirenz/Emtricitabine/Tenofovir disoproxil Krka (efavirenz / emtricitabine / tenofovir disoproxil), for the treatment of HIV infection.
Negative opinion on new medicine
The CHMP adopted a negative opinion for Aplidin (plitidepsin). Aplidin was expected to be used to treat multiple myeloma. For more information on this negative opinion, please see the questions-and-answers document in the grid below.
Three recommendations on extensions of therapeutic indication
The Committee recommended extensions of indications for Taltz, Truvada and Yervoy.
Outcome of review on mycophenolate
The CHMP concluded that current evidence does not indicate a risk of malformations or miscarriages during pregnancy when the father has taken mycophenolate medicines (used to prevent rejection of transplanted organs), although the risk of genotoxicity cannot be completely ruled out. For male patients, the CHMP now recommends that either the male patient or his female partner use reliable contraception (it is no longer required that they both use contraception). For female patients, the risk is unchanged.
For more information please see the public health communication in the grid below.
Withdrawal of application
The application for an initial marketing authorisation for Qizenday (biotin) was withdrawn. This medicine was intended to be used for the treatment of progressive multiple sclerosis. A questions-and-answers document on this withdrawal is available in the grid below.
Agenda and minutes
The agenda of the December 2017 meeting is published on EMA's website. Minutes of the November 2017 CHMP meeting will be published in the coming weeks.
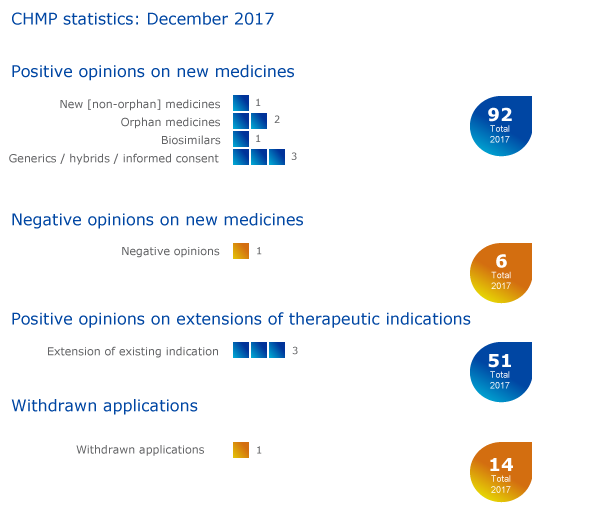
CHMP statistics
Key figures from the December 2017 CHMP meeting are represented in the graphic below.
More information on all other outcomes of the CHMP December 2017 meeting is available in the grid below.
1As always at time of approval, these orphan designations will now be reviewed by EMA's Committee for Orphan Medicinal Products (COMP) to determine whether the information available to date allows maintaining the medicines' orphan status and granting the medicines ten years of market exclusivity.
CHMP statistics: December 2017
Positive recommendations on new medicines
| Name of medicine | Alofisel |
|---|---|
| INN | darvadstrocel |
| Marketing-authorisation applicant | Tigenix, S.A.U. |
| Therapeutic indication | Treatment of complex perianal fistulas in patients with Crohn's disease |
| More information | CHMP summary of positive opinion for Alofisel
Press release: New medicine to treat perianal fistulas in patients with Crohn's disease |
| Name of medicine | Crysvita |
|---|---|
| INN | burosumab |
| Marketing-authorisation applicant | Kyowa Kirin Limited |
| Therapeutic indication | Treatment of X-linked hypophosphataemia |
| More information | CHMP summary of positive opinion for Crysvita
Press release: New medicine for rare bone disease |
| Name of medicine | Ozempic |
|---|---|
| INN | semaglutide |
| Marketing-authorisation applicant | Novo Nordisk A/S |
| Therapeutic indication | Treatment of type 2 diabetes |
| More information | CHMP summary of positive opinion for Ozempic |
Positive recommendation on new biosimilar medicine
| Name of medicine | Herzuma |
|---|---|
| INN | trastuzumab |
| Marketing-authorisation applicant | Celltrion Healthcare Hungary Kft. |
| Therapeutic indication | Treatment of breast and gastric cancer |
| More information | CHMP summary of positive opinion for Herzuma |
Positive recommendation on new hybrid medicine
| Name of medicine | Alkindi |
|---|---|
| International non-proprietary name (INN) | hydrocortisone |
| Marketing-authorisation applicant | Diurnal Ltd |
| Therapeutic indication | Replacement therapy of adrenal insufficiency in infants, children and adolescents |
| More information | CHMP summary of positive opinion for Alkindi
Press release: First paediatric medicine to treat rare hormonal disorder |
Positive recommendation on new generic medicine
| Name of medicine | Anagrelide Mylan |
|---|---|
| INN | anagrelide |
| Marketing-authorisation applicant | Mylan S.A.S. |
| Therapeutic indication | Reduction of elevated platelet counts in at risk essential thrombocythaemia patients |
| More information | CHMP summary of positive opinion for Anagrelide Mylan |
| Name of medicine | Efavirenz/Emtricitabine/Tenofovir disoproxil Krka |
|---|---|
| INN | efavirenz / emtricitabine / tenofovir disoproxil |
| Marketing-authorisation applicant | KRKA, d.d., Novo mesto |
| Therapeutic indication | Treatment of HIV infection |
| More information | CHMP summary of positive opinion for Efavirenz/Emtricitabine/Tenofovir disoproxil Krka |
Negative recommendation for new medicine
| Name of medicine | Aplidin |
|---|---|
| INN | plitidepsin |
| Marketing-authorisation applicant | PharmaMar |
| Therapeutic indication | Treatment of multiple myeloma |
| More information | (published on 20/12/2017) |
Positive recommendations on extensions of indications
| Name of medicine | Taltz |
|---|---|
| INN | ixekizumab |
| Marketing-authorisation holder | Eli Lilly Nederland B.V. |
| More information | CHMP post-authorisation summary of positive opinion for Taltz (II-09) |
| Name of medicine | Truvada |
|---|---|
| INN | emtricitabine / tenofovir disoproxil |
| Marketing-authorisation holder | Gilead Sciences International Limited |
| More information | CHMP post-authorisation summary of positive opinion for Truvada (II-135) |
| Name of medicine | Yervoy |
|---|---|
| INN | ipilimumab |
| Marketing-authorisation holder | Bristol-Myers Squibb Pharma EEIG |
| More information | CHMP post-authorisation summary of positive opinion for Yervoy (II-44) |
Public-health recommendation
| Name of medicine | Mycophenolate |
|---|---|
| More information | Mycophenolate: updated recommendations for contraception for men and women |
Withdrawal of application
| Name of medicine | Qizenday |
|---|---|
| INN | biotin |
| Marketing-authorisation applicant | Medday Pharmaceuticals |
| More information | Questions and answers on the withdrawal of the marketing authorisation application for Qizenday (biotin) |