This content applies to human medicines only.
The product management service (PMS) and substance management service (SMS) manage two of the four domains of substance, product, organisation and referentials (SPOR) master data in pharmaceutical regulatory processes. SPOR data management services facilitate the reliable exchange of medicinal product information in a robust and consistent manner.
The SPOR services support the implementation in the European Union (EU) / European Economic Area (EEA) of the standards of the International Organisation for Standardisation (ISO) for the identification of medicinal products (IDMP).
The PMS and SMS build on the data foundations of the referentials management service (RMS) and organisations management service (OMS), which EMA launched in June 2017.
For more information, see:
PMS and SMS implementation process
The implementation process of PMS and SMS follows a step-wise approach.
The first release covered a subset of ISO IDMP data fields. Later releases will see the standards fully implemented in the EU.
The first release of the SMS, in 2019, enabled users to request the registration of a new substance term or the update of an existing substance term through EMA Service Desk. This allows EMA to manage the substance data. Future releases of SMS will include synchronising SMS with the European substance reference system (EU-SRS) database and delivering an SMS user interface.
The first release of the PMS covers a subset of the authorised medicinal product part of the ISO IDMP standards. As part of this project, the new ISO IDMP compatible data submission format (HL7 FHIR) replaces the current data submission format, the eXtended EudraVigilance Product Report Message (XEVPRM).
Future PMS releases will implement other product data elements of the authorised medicinal product and the investigational medicinal product part of the ISO IDMP standards.
Data standard for information exchange
The draft international messaging standard known as Fast Healthcare Interoperability Resources (FHIR, pronounced “fire”) was endorsed as the basis for the application programming interface (API) for PMS.
FHIR will be the data standard that supports the exchange of information about medicinal products, substances, and related referential data in the European medicines regulatory network.
EMA, the European medicines regulatory network and other worldwide regulatory agencies are working with Health Level Seven (HL7) to incorporate the ISO IDMP standards into the FHIR specification.
For more information, see:
Data-centric target operating model
The target operating model (TOM) shows how product data can be re-used in regulatory processes and applications.
EMA is working to ensure that the technical components and business processes align with regulatory activities. This will ensure data quality and consistency across the PMS, national data systems and pharmaceutical companies’ data systems.
EMA gradually implements changes to TOM in several steps. The complexity of developing and implementing a data-centric business process that aligns with regulatory activities and TOM's technical components requires this approach.
As a result, transition periods when EMA uses multiple systems in parallel are unavoidable, even though this is inconvenient for regulators and applicants.
Infographic - PMS data process
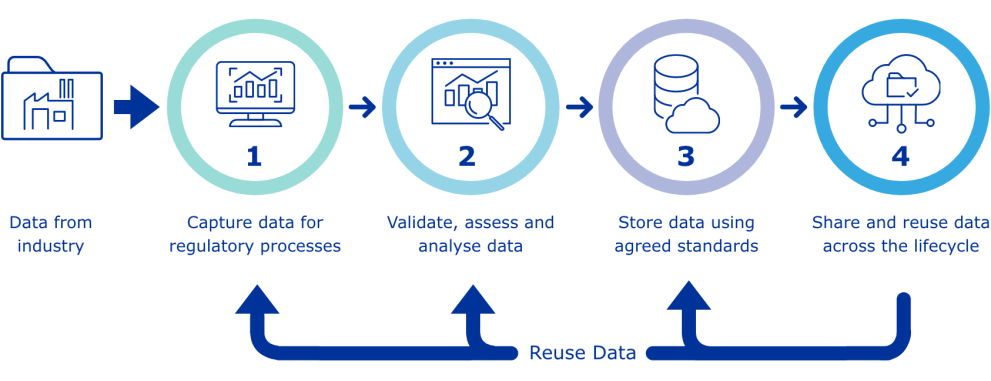
Data from the pharmaceutical is used to:
- Capture data for regulatory processes
- Validate, assess and analyse data
- Store data following agreed standards
- Share and reuse data across the medicine lifecycle
Point 1 through 4 are based on reusing data
Electronic Application Forms (eAF)
EMA's electronic application forms (eAF) facilitate capturing data for regulatory processes.
EMA made available the web-based version of the variation PDF eAF for human centrally authorised products (CAPs) and non-CAPs. It is available in the product lifecycle management portal
EMA will also replace forms supporting other regulatory procedures, such as initial marketing authorisation. This update simplifies the process of entering product data and aligns with EMA’s broader data-centric approach.
The PMS supports the implementation of the eAFs by making ISO IDMP-compatible product data available for both CAPs and non-CAPs. It is also improving the technical components needed to reflect authorised data in the PMS.
PMS and Data Management
The PMS database will provide pre-existing authorised product data. In this way, the PMS database ensures standardised data can be reused across procedures, supporting both regulatory authorities and pharmaceutical companies. The PMS makes ISO IDMP-compatible product data available for CAPs and non-CAPs.
The relevant medicine regulatory authority assesses and manages the data submitted in the dossier (and application form). Standardised product data facilitates this process.
Compliance and Reusability
The PMS stores and makes available product data and relevant documents. Once fully implemented, the PMS will fulfil the legal requirements of Article 57 of Regulation 726/2004 with its data and documentation
Regulators and pharmaceutical companies can re-use authorised product data and documents stored in the PMS in regulatory activities between them.
Infographic - PMS customer centricity
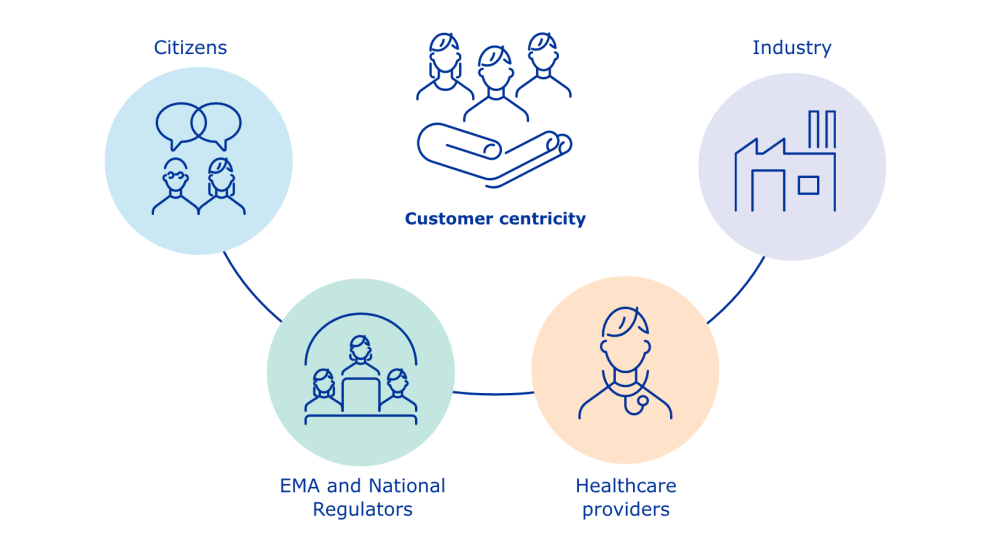
Customer centricity focuses on:
- Citizens
- EMA and national regulators
- Healthcare providers
- Pharmaceutical industry
PMS implementation
EMA and the European medicines regulatory network have agreed on a phased PMS implementation.
Select the expandable panels below for more information on the different implementation phases.
The product data preparatory phase focused on getting the target operating model ready. This ensured compliance with ISO IDMP standards.
EMA completed this phase in March 2024. It did so by:
- launching the referentials management service (LMS) and organisation management service (OMS)
- migrating data from existing systems like SIAMED and XEVMPD to PMS
The PMS user interface (PUI) aims to enable registered industry and network users to view and edit medicinal product data directly on the product lifecycle management (PLM) portal.
It provides public access to limited products data, following the guidelines in EU IG Chapter 5 and its Annex A.
EMA is gradually releasing the edit functionality. This allows users to edit limited datasets of elements of non-centrally authorised products (CAPs) for marketing authorisation holders.
Non-CAPs include products that regulatory authorities have authorised through the mutual recognition procedure (MRP), decentralised procedure (DCP), and national procedure (NAP).
In September 2024, EMA released non-CAPs in read-only mode on PUI.
The PUI went live in May 2024 in read-only mode.
For more information, see:
The PMS application programming interface (API) allows registered industry and network users to view and edit medicinal product data directly through their database systems.
Registered industry and network users can use this interface to view both CAPs and non-CAPsthat are compliant with ISO IDMP standards.
EMA is gradually releasing the edit functionality, allowing registered users to edit specific datasets related to non-CAPs for MAHs.
A roadmap is available below providing a visual overview of the PMS implementation.
How to access PMS
Access PMS User Interface (PUI)
Industry and regulator users must create an active EMA user account to access the PUI.
The product lifecycle management portal provides a user guide with step-by-step instructions for PUI registration.
The general public can access a limited authorised data set through the 'human medicinal product overview report' available on the PUI portal.
The EU IG Chapter 5 outlines the principles that regulate access to PMS.
Access the PUI portal in the link available below:
Access PMS Application Programming Interface (API)
Industry and regulator users need to have an active EMA user account to access the PMS API.
Please consult the EU IDMP implementation guide chapter 1 – section 3.2.1. for step-by-step instructions on how to register and get access to PMS API.
Currently, the general public cannot access a limited authorised data set through the PMS API.
Key actions for PMS users
Key actions for marketing authorisation holders (MAH)
- Submit pack sizes in XEVMPD according to chapter 3.II instructions, when applicable.
- Verify that the data submitted to XEVMPD/SIAMED displays correctly in PMS. This applies to both CAPs and non CAPs (through the PUI or PMS API). If you notice discrepancies or have doubts about the data, refer to Chapter 7 and Chapter 9 of the EU IDMP Implementation Guide (IG) and the PMS FAQ document. If further clarification is needed, MAHs should open an EMA service desk ticket following the instructions in the “support” section of the PUI navigation guide.
- For non-CAPs, start gathering structured data on pack sizes and manufacturers in preparation for submissions through PMS. For more information, refer to the supporting material on the hosted PMS webinar on the PUI edit functionalities for industry users event page.
Additional points:
- MAHs should begin submitting and maintaining structured data for all non-CAPs impacted by the “Union list of critical medicines” by the final deadline of December 2025
- Users can identify the impacted products by following the instructions in the ULCM section
- MAHs can also submit and maintain structured data for all other products, not impacted by the “union list of critical medicines,” by December 2026
For more information, see:
- Product Management Service (PMS) webinar on Product User Interface (PUI) edit functionalities for industry users
- Union list of critical medicines
- PMS guidance documents
Key actions for National Competent Authorities (NCA)
- Match PMS data on medicinal products and packages in the API or PUI with national systems and investigate any discrepancies in the identified product data
- Request the EMA data mapping service, available until December 2025
- Submit product data for data mapping services via EMA SD by the 5th of May 2025, find instructions on the EU-NTC platform
- Optionally, review the structured non-CAPs data that MAHs submit in PMS
For more information, see:
Mandatory submission of medicine data
Information helping marketing authorisation holders and national competent authorities to map their products in EMA's product management service (PMS) is available to registered users with full access to the Product Lifecycle Management portal (PLM):
This helps them report data on the supply and demand of medicines to the European Shortages Monitoring Platform. It is part of their mandatory reporting requirementsand is meant to help prevent, detect and manage human medicine shortages.
Guidance on how to access the dynamic product reports that contain this information is also available on the PLM portal:
EU IDMP Implementation Guide
The EU IDMP Implementation Guide (EU IG) for the submission of data on medicinal products defines the implementation requirements of the ISO IDMPstandards for the EU.
The EU IG is the basis for submitting and exchanging medicinal product data in the EU.
Its purpose is to enable stakeholders to prepare for the implementation of ISO IDMP standards in EU and it provides information on the following:
- Timelines
- Requirements
- Process
- Technical specification
- Data elements
- Associated business rules
The EU IG is composed of the chapters below.
EMA updates the EU IG chapters are regularly to reflect progress in PMS and support the European medicines regulatory network in preparing for its implementation.
EU IG v2.0 and further minor releases, including EU IG v2.1 and EU IG v2.1.1, mainly support the implementation of PMS Step 1 and data submission on medicinal products authorised through the centralised procedure.
They provide:
- detailed guidance on registration requirements as well as information on product data access;
- clarification on the concepts of the target operating model of PMS;
- clarification on the processes for submission, exchange or validation of medicinal product information;
- clarification on the data elements and the applicable business rules for electronic submission of information on medicinal products for human use;
- practical examples to support SPOR users in correctly structuring their product data in situations where the direct application of ISO IDMP may be complex.
This information enables the European medicines regulatory network to prepare for the submission of data on all medicinal products for human use authorised in the EU. It offers a basis for practical preparation activities, such as:
- performing proofs-of-concept on end-to-end processes involving the generation and submission of FHIR messages, validation and interaction with eCTD;
- testing the use cases.
Close collaboration between all stakeholders is crucal in these preparation activities, including industry, software vendors and regulators.
The European medicines regulatory network has agreed a phased release plan for the EU Implementation Guide. EMA is releasing several versions to reflect the details of the latest agreements available and enhance quality.
To minimise the impact on implementation, EU IG v2.1 focuses on enhancing data-related aspects to provide visibility of data elements, business rules, data transformation and required data collection, whereas EU IG v2.2 will focus mostly on process clarifications for the electronic submission of authorised medicinal product data in PMS.
EU IG v2.1 contains minor updates to the data elements that should be reported to PMS, further details on the RMS lists that are needed and more examples. EU IG v2.1 also contains new data elements that required to support the PMS target operating model foreseen in PMS Step 2. These new data elements are part of ISO IDMP standards, have optional or conditional submission and address future integration with eAFs and reporting on marketing status to support the handling medicine shortages.
EU IG v2.1.1. provides:
- further details on the data elements introduced to support the new web-based forms introduced by the DADI project;
- updated business rules and FHIR paths for these data elements
- updated details on the applicable RMS lists;
- further examples and clarifications;
- details on migration rules applied between EMA's internal database, SIAMED and PMS for centrally authorised products
Although the data fields and specifications presented in EU IG v2.1.1 should remain stable, some business rules may be subject to minor modifications in future releases of the guide.
While no substantial changes are expected to the submission process, further details may be added, in version 2.2, particularly with regards to data migration and enrichment of data.
EMA will publish more chapters as part of the guide's next major release, EU IG v3, to help the European medicines regulatory network prepare for Step 2, the last step of product data implementation.
The specification of the SPOR API reflected in Chapter 6 is subject to development and testing. As with any software, it may evolve over time and will be subject to change control.
Both this guide and the API can be expected to evolve with understanding of business processes and requirements and application of technological improvements, although no significant changes are expected.
EU Substance Registration System: EMA's role
As of January 2023, EMA hosts and maintains the EU Substance Registration System (EU-SRS) that gathers scientifically sound data on substances used in medicines.
These data include defining characteristics of substances, molecular structures, and amino acid sequences and relationships.
The EU-SRS covers a variety of use cases, such as:
- providing insight on the characteristics of a substance;
- supporting assessors in the areas of quality, safety and pharmacovigilance;
- helping introduce a structured data approach for regulatory submissions.
The Substances Validation Group maintains the actual substance data. This group brings together experts from national competent authorities in EU countries.
The German Federal Institute for Drugs and Medical Devices (BfArM) previously maintained the EU-SRS.
For more informatin, see:
How to stay informed on PMS
For the latest news, updates and announcements on the product management service (PMS), see:
For user guides, QandA documents, recordings of training sessions and the union list of critical medicines, see:
To explore past and upcoming PMS events on EMA's website, including presentations, agendas, recordings and registration links, see: