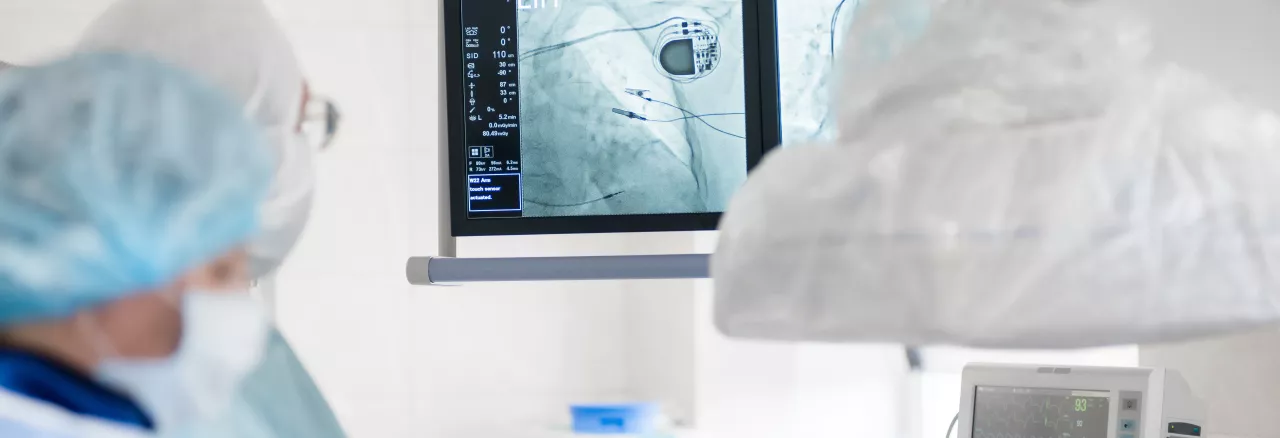
Advice and guidance for manufacturers

Regulators, healthcare professionals and consumers working together

How the CVMP protects animal and public health
CHMP recommends eight new medicines for approval in the EU

Health and environment agencies collaborate under One Health approach

Platform enables reporting on supply, demand and availability of medicines

Milestone highlights EMA's changing role in Europe
Find medicine
What's new
FAQs
Latest news
Manufacturers can request advice on their medical device clinical development programme

Clinical Trials Information System now supports submission, assessment and oversight of all trials in the EU
EMA opinion for ivermectin and albendazole supports global efforts to eliminate widespread infections

Eight new medicines recommended for approval; one positive opinion for a medicine intended for use outside the EU
The review of Vimkunya was expedited because of public health interest

The extensive use of azole fungicides (azoles), particularly in some agricultural and horticultural practices, can increase the risk of Aspergillus fungi developing resistance to essential antifungal treatments. This significant finding is highlighted in a report by the five EU health and environment agencies, with support from the European...