The Veterinary Medicinal Products Regulation (Regulation (EU) 2019/6) applies from 28 January 2022.
For more information, including guidance on how to register, see:
Separate guidance is also available on Variations requiring assessment.
List of variations not requiring assessment
Guidance on variations not requiring assessment is available in the implementing regulation linked below. This includes the list of variations not requiring assessment, the requirements and conditions to be met, and the documents that marketing authorisation holders need to submit for each variation type.
- Commission Implementing Regulation (EU) 2021/17 of 8 January 2021 establishing a list of variations not requiring assessment in accordance with Regulation (EU) 2019/6 of the European Parliament and of the Council
- Commission Implementing Regulation (EU) 2023/997 of 23 May 2023 amending Implementing Regulation (EU) 2021/17 establishing a list of variations not requiring assessment in accordance with Regulation (EU) 2019/6 of the European Parliament and of the Council
- Commission Implementing Regulation (EU) 2024/916 of 26 March 2024 amending Implementing Regulation (EU) 2021/17 establishing a list of variations not requiring assessment in accordance with Regulation (EU) 2019/6 of the European Parliament and of the Council
Questions and answers
Commission Implementing Regulation (EU) 2021/17 establishes a list of VNRA. Such minor variations have only a minimal impact, or no impact at all, on the quality, safety or efficacy of the veterinary medicinal product, and do not require prior approval before implementation.
The MAH shall record the variation attaching, as applicable, any supporting documentation and the product information in all EU languages, in the UPD within 30 days following the implementation of that change.
The Annex to the Implementing Regulation (EU) 2021/17, as amended by Regulation (EU) 2023/997, clarifies the conditions which must be met in order for a specific change to be considered a VNRA, including the required supporting documentation.
Meaning of “implementation” for VNRA
For quality changes, implementation is the date when the company makes the change in its own Quality System. This can be a past or future date.
This interpretation allows companies to manufacture conformance batches and generate any needed stability studies to support a VRNA before recording the variation in the UPD1 because the change will not be made in their own Quality System until these data are available.
For the introduction of the summary of the pharmacovigilance system master file or for changes to the elements included in the summary of the pharmacovigilance system master file, e.g. name or address or contact details of a qualified person for pharmacovigilance (QPPV), change in the pharmacovigilance system master file (PSMF) location, ‘implementation’ is when the company makes the change in its summary of the pharmacovigilance system master file and reflects this change in its PSMF.
For product information, ‘implementation’ is when the company internally approves the revised product information in its own quality system. The revised product information will then normally be used as a basis for the next packaging run.
1For example, the VNRA for a change in the batch size of the finished product for a more than 10-fold increase compared to the originally approved batch size for an immediate release, solid oral pharmaceutical form requires stability data on at least one pilot scale batch.
References
In accordance with Articles 64 and 65 of Regulation (EU) 2019/6, grouping and worksharing procedures, respectively, do not apply to VNRA. Therefore, VNRA cannot be included in groupings or worksharing of variations requiring assessment (VRA), even if they are consequential or related to the VRA included in such procedures. In such cases, the consequential or related variations of both types could be submitted and coordinated in a way that approval of the variations at the same time is possible, however it is considered that this would actually increase administrative burden for the MAH. As such, it is advised that VNRA consequential to VRA are submitted after approval of the VRA, please refer to the separate Q&As on VRA.
For VNRA, the UPD allows MAH to record “technical” groupings. This may be a grouping of an identical change for multiple products at the same time, by including several VNRA within one submission. It may also be a grouping of several changes classified as VNRA for the same product, but other combinations are possible.
Products, for UPD purposes, are defined as combination of name, substance, strength and pharmaceutical form as illustrated below (with the hypothetical names ‘Sample’ and ‘Example’):
- Sample (Marketing Authorisation)
- Sample, benazepril hydrochloride, 5 mg, tablets
- Sample, benazepril hydrochloride, 20 mg, tablets
- Example (Marketing Authorisation)
- Example, meloxicam, 20 mg/ml, solution for injection
- Example, meloxicam, 40 mg/ml, solution for injection
For technical grouping of variations concerning one marketing authorisation, all VNRA must be declared in the UPD. The supportive documentation for all variations concerned should be submitted as one integrated package.
For technical grouping of VNRA concerning several marketing authorisations, all VNRA must be declared in the UPD for all veterinary medicinal products. The supportive documentation for all variations concerned should be submitted as one integrated package. Product-specific documentation should be provided in separate files and MAH should ensure that file names allow easy identification of the related products.
- Several VNRA affecting one veterinary medicinal product
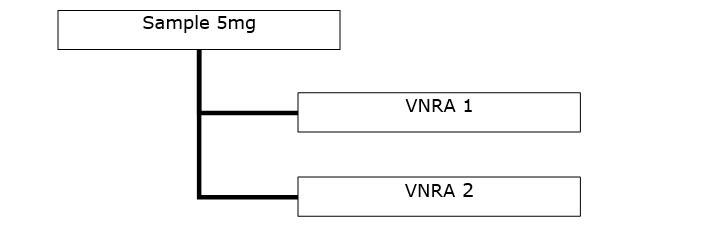
- One VNRA affecting several veterinary medicinal products from the same marketing authorisation.
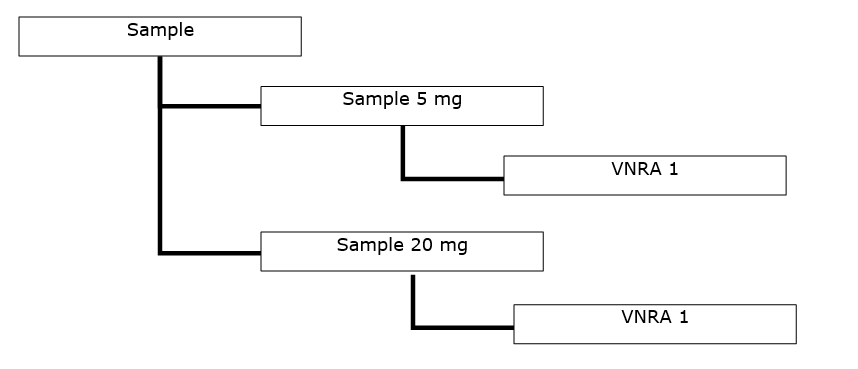
- Several VNRA affecting several veterinary medicinal products from the same marketing authorisation. The variations are the same for all veterinary medicinal products and are submitted to the same competent authority.
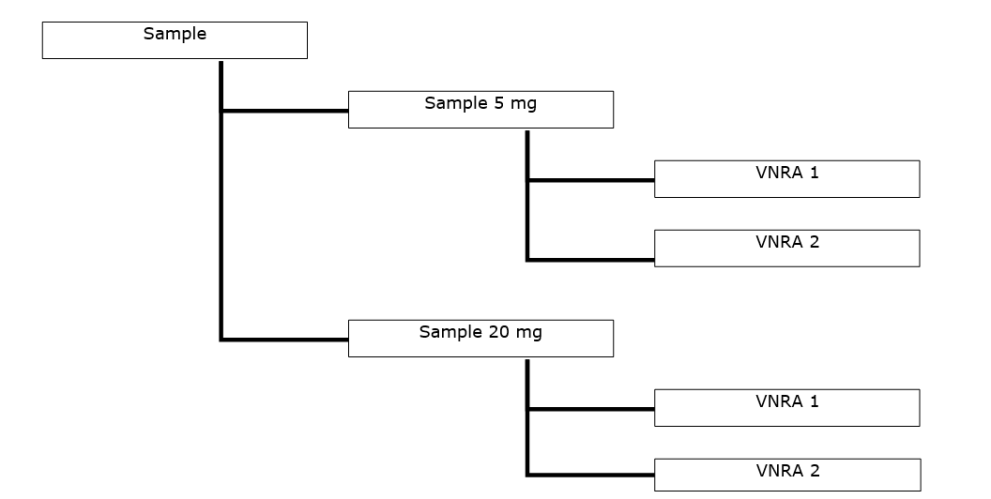
- One VNRA affecting several veterinary medicinal products approved under different routes of authorisation. The variations are the same for all veterinary medicinal products and are submitted to the different competent authorities. The VNRA will be accepted or rejected by the relevant competent authority; EMA accepts or rejects VNRA for centrally authorised products, on behalf of EC.
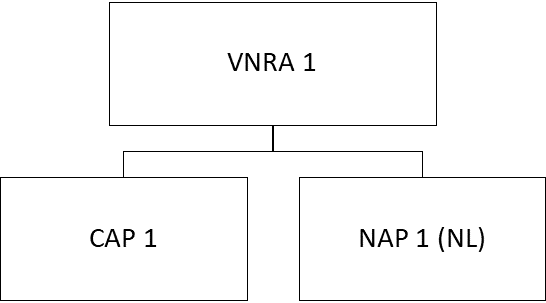
- One VNRA affecting several veterinary medicinal products from different marketing authorisations of the same MAH (or if applicable, from different MAHs).
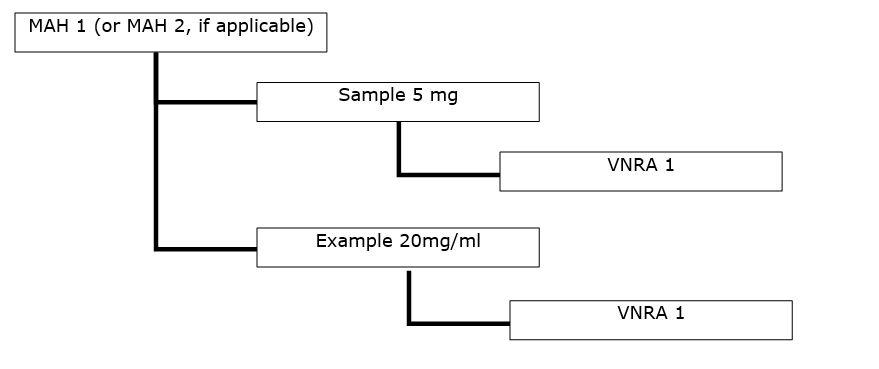
- Several VNRA affecting several veterinary medicinal products from different marketing authorisations from the same MAH (or if applicable, from different MAHs). The variations are the same for all veterinary medicinal products and are submitted to the same competent authority.
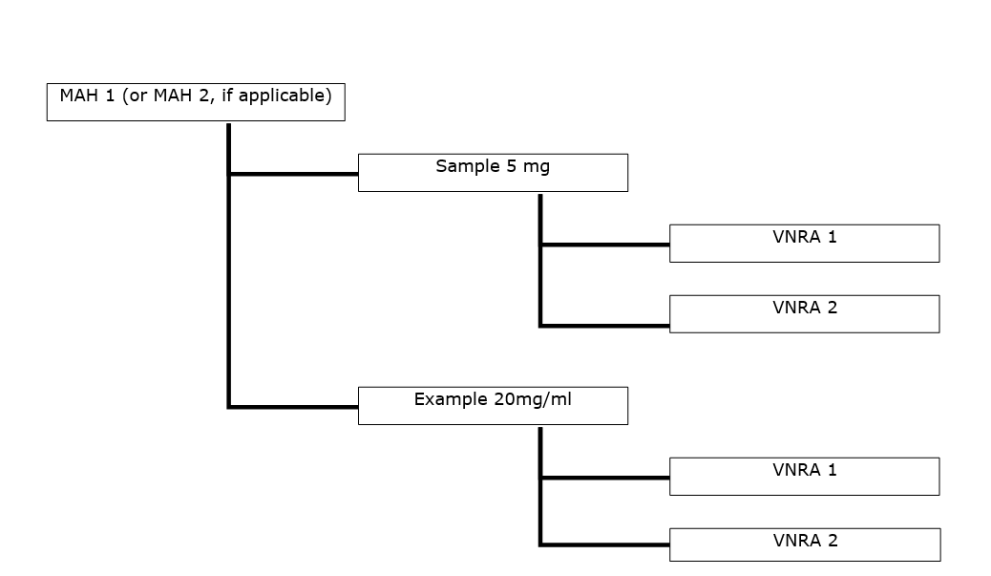
- Several VNRA affecting several veterinary medicinal products approved under different routes of authorisation. The variations are the same for all veterinary medicinal products and are submitted to the relevant different competent authorities; EMA accepts or rejects VNRA for centrally authorised products, on behalf of EC.
- Important: although technically possible, this case is strongly discouraged as it would lead to great obstructions and possible delays in processing VNRA’s.
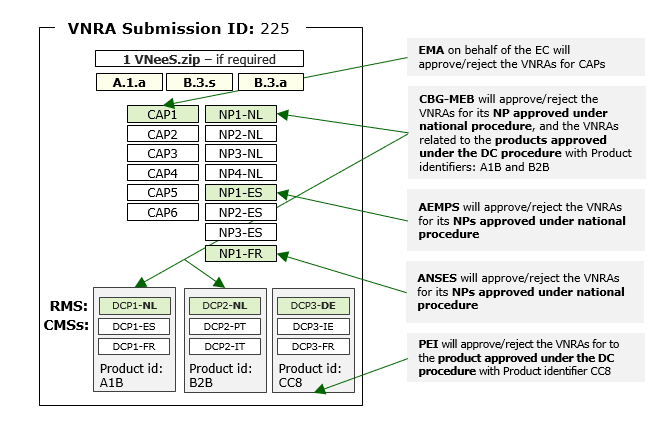
- Please note that in this case the VNeeS structure applies, and should be presented as per example below:

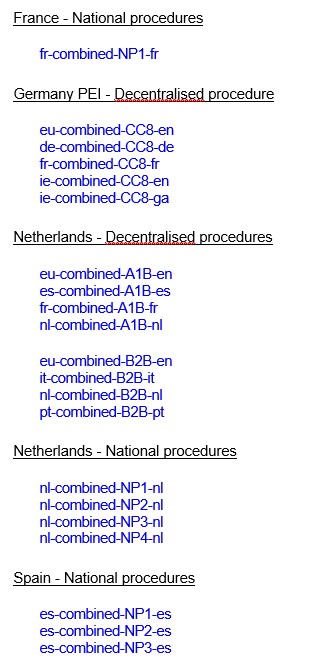
It must be noted, however, that when submitting VNRA as part of a technical grouping, the legal deadlines for submission of each variation should be respected, i.e. a VNRA should always be recorded in the UPD within 30 days following the implementation of that VNRA.
References
- Commission Implementing Regulation (EU) 2021/17
- Procedural advice for requests for the classification of variations not already listed in Commission Implementing Regulation (EU) 2021/17 or EMA/CMDv Guidance on the details of the classification of variations requiring assessment according to Article 62 of Regulation (EU) 2019/6
- EMA website on the Union Product Database
- EMA account management
- Q&A on VRA
The Agency will review the VNRA and its documentation, usually within 30 calendar days, following recording of the procedure in UPD, by the MAH. The rapporteur or co-rapporteur are not involved in the review of VNRA.
The same principle applies whether a single VNRA or a technical grouping of VNRA is being recorded in the UPD.
A VNRA should be recorded in the UPD by selecting and filling in the following UPD fields: classification code, concerned product(s), submission comments, date(s) of implementation, confirmation of compliance with conditions.
Supporting documentation as specified in the Annex to the Commission Implementing Regulation (EU) 2021/17, as amended, should be included in a zip-file with VNeeS format, with the appropriate headings and numbering as per Commission Delegated Regulation (EU) 2021/805 amending Annex II of Regulation (EU) 2019/6. MAHs are advised to run the VNeeS checker on the VNeeS file and include the validation report in the Add-info folder of the VNeeS file.
A cover letter may be included in the VNeeS file to give further information on the variation.
The precise scope of the VNRA, details on former and new data and a mention if the VNRA is consequential to a VRA should be included in the UPD “submission comments” field. If information on former and new data is too extensive, this can be also provided as an attachment to be included in the zip-file, under the 1a-admin-info folder. The “former and new data” table should clearly identify the relevant dossier sections (to the lowest level possible) in support of each variation. Omission of clear information on the precise scope of the VNRA in the UPD “submission comments” field can lead to rejection of the variation.
For variations that are technical groupings of VNRA affecting several marketing authorisations, cross-references to any documentation submitted for another veterinary medicinal product cannot be accepted. Each submission must have the required documentation, in VNeeS format, for each marketing authorisation concerned by the grouped VNRA.
If a variation is submitted because it has been requested as an outcome arising from another pre-/
post-authorisation procedure (including as a result of signal detection or of a post-authorisation measure (PAM), e.g. specific obligation, recommendation), this should be clearly mentioned in the UPD ”submission comments” field for the resulting variation, identifying the related EMA procedure number from which the variation arose. A copy of the request should be included in the VNeeS file.
In case the reference number for the PAM has not been confirmed by the Agency, a description of the commitment/measure is sufficient at time of recording the VNRA in the UPD.
If applicable, the revised product information (summary of product characteristics (Annex I), Annex II, labelling (Annex IIIA) and/or package leaflet (Annex IIIB)) should be provided as a full set of annexes per EU language (Word highlighted and PDF clean). (See also “When do I have to submit revised product information? In all languages?”)
It should be noted that the responsibility for the quality of the submitted documentation lies with the MAH and is crucial to the overall process. The MAH is responsible for ensuring that the VNRA complies fully with the requirements, meeting the requirements (conditions and documents to be provided) as specified in Commission Implementing Regulation (EU) 2021/17.
VNRA are intended to provide for a simple, rapid and efficient procedure for minor changes. The MAH should be aware that the submission of redundant information, or a confusing dossier presentation will not facilitate smooth handling of procedures. Similarly, deficient and missing documentation will lead to rejection of the variation.
For more information, including links to guidance on registration with the UPD, see the Veterinary eSubmission website and EMA website on the Union Product Database.
All submissions should be made in accordance with the guideline on e-submissions.
References
- Regulation (EU) 2019/6
- Commission Delegated Regulation (EU) 2021/805
- Commission Implementing Regulation (EU) 2021/17
- Procedural advice for requests for the classification of variations not already listed in Commission Implementing Regulation (EU) 2021/17 or EMA/CMDv Guidance on the details of the classification of variations requiring assessment according to Article 62 of Regulation (EU) 2019/6
- Dossier requirements for submission of marketing authorisation and maximum residue limit (MRL) applications to the European Medicines Agency (EMA) and to members of the Committee for Medicinal Products for Veterinary use (CVMP)
- Veterinary eSubmission website
- eSubmission Gateway and Web Client information website
The Agency will review the VNRA usually within 30 calendar days following it being recorded in the UPD. The Agency will check the correctness of the information recorded in the UPD, the presence of the required documentation and compliance with the specified conditions in accordance with the Annex to the Commission Implementing Regulation (EU) 2021/17, as amended.
• Start of Agency check: Day +1 following recording of VNRA in UPD
• Review outcome (Approval/Rejection): usually by Day + 30
In most cases, the MAH will be informed of the outcome of the VNRA by Day 30; this will happen in the UPD. Where one or several VNRA scopes are submitted as part of one VNRA submission, the MAH will be clearly informed which variation(s) have been accepted or rejected.
Where the outcome of the procedure is favourable and the terms of the Commission Decision granting the marketing authorisation (including Annexes thereto) require amendment, the Agency will inform the Commission accordingly. See “15. How and when will the updated product information become part of the Marketing Authorisation?”
VNRA can be implemented prior to submission in the UPD. However, in case a VNRA has been rejected, the MAH should cease applying the rejected variation(s), unless a corrected VNRA can be submitted within a reasonable time after implementing the change. Please refer to “7. What should I do in case my VNRA is rejected?” for further details.
References
- Regulation (EU) 2019/6
- Commission Implementing Regulation (EU) 2021/17
- Procedural advice for requests for the classification of variations not already listed in Commission Implementing Regulation (EU) 2021/17 or EMA/CMDv Guidance on the details of the classification of variations requiring assessment according to Article 62 of Regulation (EU) 2019/6
No, VNRA are not subject to worksharing procedures.
VNRA will be rejected when not all of the requirements for the VNRA are met, e.g. not all of the conditions are met or when the submitted documentation as required by Commission Implementing Regulation (EU) 2021/17, as amended, is deficient. In such case, the MAH shall cease to apply the rejected changes, unless a corrected VNRA can be submitted within a reasonable time after implementing the change.
In the case of a rejection of a VNRA, e.g. when the requirements in relation to the documentation or conditions are not met, and where the change should actually be implemented by means of a variation requiring assessment, it is the MAH’s responsibility to judge whether the rejected VNRA has an impact on the quality, safety or efficacy of the veterinary medicinal product. If this is the case, the MAH has to take appropriate action..
The Agency may ask the MAH to complete a suspected quality defect notification form and provide a Risk Assessment report on the impact of the product on the market via e-mail to qdefect@ema.europa.eu within 7 calendar days from the date of the rejection letter. Such requests are expected to be very exceptional. The MAH should follow the instructions under Quality Defects.
References
- Regulation (EU) 2019/6
- Commission Implementing Regulation (EU) 2021/17
- Procedural advice for requests for the classification of variations not already listed in Commission Implementing Regulation (EU) 2021/17 or EMA/CMDv Guidance on the details of the classification of variations requiring assessment according to Article 62 of Regulation (EU) 2019/6
- Quality defects and recalls
VNRA are not subject to fee payment.
Any changes in the number of units of a veterinary medicinal product will trigger a different EU number (UPD - presentation number).
Differentiation should be made between the addition of a presentation where the two presentations will co-exist on the market on a long-term basis versus a replacement of a presentation where the new presentation will replace the previous one (it is expected that for a certain period of time the two presentations will co-exist on the market until the stock of the previous presentation runs out).
In principle, a replacement of one presentation by another presentation does not trigger a new EU number, unless the number of units of veterinary medicinal product is changed.
Examples of changes in presentations for replacement, not triggering a new EU number (this is not an exhaustive list):
- Replacement of the primary or secondary packaging,
- Change in units per blister (without change to the total number of units per pack).
Examples of changes in presentations for replacement, triggering a new EU number (this is not an exhaustive list):
- 30 to 60 tablets,
- 2 prefilled syringes containing the veterinary medicinal product instead of 1 prefilled syringe.
In case of an addition of a presentation, as the presentations will co-exist on the market, two packs with different contents cannot be covered by the same EU number and will be considered as different presentations.
Changes in the number of any unit (not restricted to the veterinary medicinal product) or changes in the specifications of any unit (not restricted to the veterinary medicinal product) contained in the pack will trigger a new EU number.
Examples of changes that will trigger new EU numbers (this is not an exhaustive list):
- Introduction of an alternative syringe of different volume,
- Introduction of an alternative immediate (primary) packaging made from a different material,
- Introduction of an alternative shape/dimension of a pharmaceutical form.
If you have any questions on any upcoming submission, please contact the Agency via Service Now by selecting Veterinary Regulatory > Post-Authorisation-Vets.
In the specific case of a VNRA for an additional presentation, the new EU marketing authorisation number (UPD - presentation number) should be requested from the Agency before implementation.
The request should be sent to the Agency via Service Now by selecting Veterinary Regulatory > Post-Authorisation-Vets, with a copy to the product shared mailbox and should be made at least 5 working days in advance of the intended submission of the variation. Once a number has been allocated, this number should subsequently be included in the product information annexes submitted together with the VNRA.
Range is defined from the smallest to the biggest approved pack size for the same pharmaceutical form and strength. The pack size equals the number of units of the pharmaceutical form (e.g. tablets, sachets, ampoules, etc.) contained per outer packaging. Pack sizes not included within this range are considered to be outside of the range. For the addition of a new pack size where the number of units in the pack is within the range of the currently approved pack sizes for the strength and pharmaceutical form, applicants should submit a VNRA B.38.
For the addition of a new pack size where the number of units of the pack is outside the range of the currently approved pack sizes for the strength and pharmaceutical form, applicants should submit a VRA F.II.e.5.a.
In support of a timely introduction of new pack sizes to the market, the EMA accepts the following approach for the introduction of various pack sizes falling outside the range. The biggest or the smallest pack size per strength outside the range should be classified as VRA F.II.e.5.a. This presentation defines the new limits of the range so that any intermediate pack size for the strength and pharmaceutical form can be classified as VNRA B.38.
Some examples are provided below to illustrate the principles explained above.
Example 1
The 20 mg strength of ‘Veterinary Medicinal Product A’ currently has two approved pack sizes of 30 and 60 tablets for the pharmaceutical form ‘film-coated tablets’ and the MAH intends to apply for a new pack size of 45 tablets. The introduction of a new pack size of 45 tablets for the 20 mg strength is considered within the range of approved packs (30-60 tablets) and should be classified as a VNRA B.38.
Example 2
The 20 mg strength of ‘Veterinary Medicinal Product A’ currently has two approved pack sizes of 30 and 60 tablets for the pharmaceutical form ‘film-coated tablets’ and the MAH intends to apply for a new pack size of 90 tablets. The introduction of a new pack size of 90 tablets for the 20 mg strength is considered outside the range of approved packs (30-60 tablets) and should be classified as a VRA F.II.e.5.a.
Example 3
The 20 mg strength of ‘Veterinary Medicinal Product A’ currently has two approved pack sizes of 30 and 60 tablets for the pharmaceutical form ‘film-coated tablets’ and the MAH intends to apply for two new pack sizes of 90 and 120 tablets at the same time. The introduction of a new pack size of 120 tablets for the 20 mg strength is considered outside the range of packs and should be classified as variation VRA F.II.e.5.a. This pack size defines a new limit for the range (30-120), so that the introduction of a pack size of 90 tablets can be classified as a variation VNRA B.38.
Example 4
The 20 mg and 40 mg strengths of ‘Veterinary Medicinal Product B’ currently each have two approved pack sizes of 2 and 10 pre-filled syringes for the pharmaceutical form ‘solution for injection’. The MAH intends to apply for four new pack sizes:
- 5 and 30 pre-filled syringes for the 20 mg strength;
- 5 and 30 pre-filled syringes for the 40 mg strength.
For the 20 mg strength, the introduction of a new pack size of 5 pre-filled syringes strength is considered within the range of approved packs (2-10) and should be classified as variation VNRA B.38 and the introduction of a new pack size of 30 pre-filled syringes is considered outside the range of approved packs (2-10) and should be classified as variation VRA F.II.e.5.a.
For the 40 mg strength, the introduction of a new pack size of 5 pre-filled syringes strength is considered within the range of approved packs (2-10) and should be classified as variation VNRA B.38 and the introduction of a new pack size of 30 pre-filled syringes is considered outside the range of approved packs (2-10) and should be classified as variation VRA F.II.e.5.a.
The MAH should therefore apply to the EMA for a grouped VRA and record the VNRA in the UPD as part of a so-called ‘technical grouping’. Please refer to “Can I group the submission of VNRA? Can they be grouped with other types of variations?” for further details.
Example 5
The 50 mg strength of ‘Veterinary Medicinal Product C’ currently has two approved pack sizes of 10 and 30 tablets for the pharmaceutical form ‘film coated tablets’ and the MAH intends to apply for a multipack of 30 (3x10) tablets. The introduction of a multipack 30 (3x10) tablets for the 50 mg strength is considered within the range of approved packs (10-30) and should be classified as variation VNRA B.38.
IMPORTANT
For VRA introducing additional presentations or pack sizes, each additional presentation or pack size attracts separate fees (x additional presentations = x separate fees). Each presentation and pack size should therefore be declared as a separate variation on the variation application form under the section ‘variations included in this application’.
Changes of strength, pharmaceutical form and route of administration are to be submitted as a VRA under chapter I.
References
Please refer to the Q&A on Changing the (invented) name of a centrally authorised medicinal product.
References
Where the VNRA application affects SPC, labelling and/or package leaflet, the revised product information must be submitted as follows:
- The complete set of Annexes in all EEA languages should be provided as part of the VNeeS File variation application, in Word format (highlighted) and in PDF (clean).
- The ‘complete set of Annexes’ includes SPC (Annex I), Annex II, labelling (Annex IIIA) and package leaflet (Annex IIIB) for all strengths and pharmaceutical forms of the product concerned. The complete set of Annexes must be presented sequentially (i.e. Annex I, II, IIIA, IIIB) as one document for each official EEA language. Page numbering should start with "1" (bottom, centre) on the title page of Annex I. All Annexes should be in compliance with the ‘QRD Convention’ published on the Agency website. When submitting the full set of Annexes in PDF format, the formatting checklist, the user guide on how to generate PDF versions of the product information and Annex II of the EU Implementation Guide (Vet EU IG) on veterinary medicines product data in the Union Product Database must be followed.
- Highlighted changes in the WORD-files should be indicated via ‘Review – Track Changes’. Clean versions should have all changes ‘accepted’.
- Icelandic and Norwegian language versions must always be included.
- The Annexes provided should only reflect the changes introduced by the variation(s) concerned.
Any changes not listed in the UPD comment field (or former and new table, as applicable) will not be considered as part of the variation notification. In such cases, and in cases where any other ongoing procedures may affect the product information Annexes, the MAH is advised to contact the Agency in advance of submission or finalisation of the procedure(s) concerned. - Where the variation introduces a new EU sub-number, the new sub-number should be included in the product information texts as part of the variation application (see also “How to obtain new EU sub-numbers (presentation numbers) for a VNRA variation concerning an additional presentation (e.g. new pack-size)”?). Similarly, in case of a deletion of a pharmaceutical form/strength/pack-size(s), the product information Annexes should be provided as part of the variation application.
References
- Product information: Reference documents and guidelines
- Quality Review of Documents (QRD) convention to be followed for the European Medicines Agency QRD templates (currently under review, link will be provided once available)
- User guide on how to generate PDF versions of the product information - veterinary
- Annex II of the EU Implementation Guide (Vet EU IG) on veterinary medicines product data in the Union Product Database
For VNRA affecting the product information, i.e. the Annexes to the Commission Decision, the concerned changes in the product information will be reflected in the next Commission Decision amending the terms of the marketing authorisation following the assessment of a VRA or another regulatory procedure (e.g. referral, pharmacovigilance activity, etc). When the opinion for this VRA or other regulatory procedure is transmitted to the Commission, the changes of the VNRA will already be included in the annexes to that opinion and will consequently be reflected in the resulting Commission Decision.
A stand-alone Commission Decision to reflect a VNRA will only be adopted in exceptional cases and when duly justified by the MAH.
here a VNRA concerns several marketing authorisations, the Commission will update these marketing authorisations in due course and with one decision per marketing authorisation concerned.
VNRA do not require prior approval before implementation, i.e. they are do and tell procedures.
For VNRA affecting the product information, the date of revision of the text to be included in
- QRD template v.8.2: section 10 of the summary of product characteristics (SPC) and corresponding section 14 of the package leaflet at the time of printing1
- QRD template v.9: section 9 of the SPC and corresponding section 15 of the package leaflet at the time of printing1
should be the date of implementation of the change by the MAH. For the meaning of “implementation” see “When shall I record my VNRA?”. It is possible that the dates in the SPC and in the package leaflet are not always the same if there has been a variation that affects one and not the other.
1The date of revision of the text in both section 10 of the summary of product characteristics and in the corresponding section 14 of the package leaflet should be left blank in the full set of Annexes submitted to the Agency.
General principles
With the exceptions described below, the Agency will not accept any editorial changes as part of VNRA. This is due to the fact that VNRA notifications are administrative in nature and do not have a validation or an assessment phase.
Editorial changes should be submitted within procedures that have an administrative validation phase (e.g. VRA). This allows the appropriate review of proposed editorial changes during validation and the consequential amendment of the submission prior to assessment, if needed. The editorial changes proposed should affect the same part of the dossier concerned by the variation procedure. For example, if a variation affects Part 2.C of the dossier, editorial changes to Part 2.C can be submitted.
MAHs are reminded to follow this guidance and to ensure the high quality of variation applications in support of a timely processing of these submissions.
Editorial changes in Part 2 (quality)
If editorial changes are required and there is no upcoming procedure with a validation phase, the MAH may consider submitting these changes as a separate VNRA (B.43).
The following may be considered as examples of editorial changes to Part 2: adding headers for ease of use, reordering of existing information without changing the meaning, alignment of information among/within the sections provided the correct reference that had been previously agreed can be demonstrated (e.g. alignment of information in flow charts to process description), punctuation changes and grammar/orthographic corrections that do not alter the meaning of the text.
Examples of changes that cannot be considered editorial: removal of specification parameters or manufacturing description, update of information to bring the dossier content in line with the current manufacturing process, etc.
Proposed changes that require confirmation by the rapporteur may only be accepted when submitted within the scope of an upcoming VRA which impacts upon the corresponding section of Part 2.
Editorial changes in Parts 3 (safety & residues) and 4 (pre-clinical & clinical)
Editorial changes in Parts 3 and 4 are not foreseen. Please contact the Agency via Service Now by selecting Veterinary Regulatory > Post-Authorisation-Vets in advance of an upcoming submission.
Editorial changes to the product information in Part 1.B (SPC, labelling and package leaflet)
Should there be no upcoming regulatory procedure with a validation phase in which to include the editorial changes, these could also be submitted as a stand-alone VNRA (C.9).
Formatting changes, correction of typographical errors and/or mistakes to the English product information or other linguistic versions of the product information are considered editorial changes provided that the meaning of the text is not altered. These changes can be included within the scope of any upcoming procedure impacting on the product information and including an administrative validation phase (e.g. VRA).
Changes in the scientific content or meaning cannot be accepted as an editorial change. These changes should be classified under the scope of the relevant category as per the EMA/CMDv Guidance on the details of the classification of variations requiring assessment (e.g. VRA, G.I.4).
Proposed changes that may require confirmation by the rapporteur or linguistic review will only be accepted when submitted within the scope of an upcoming VRA under chapter G (of the EMA/CMDv Guidance on the details of the classification of variations requiring assessment) which impacts upon the product information and where linguistic review is foreseen, if applicable.
The MAH should liaise with the Agency without delay if the mistake concerns incorrect or missing important information (e.g. contra-indication or adverse event), in the English or any of the other language versions, that could affect the safe and effective use of the veterinary medicinal product and/or lead to a potential medication errors (e.g. wrong strength, wrong posology, wrong route of administration).
In cases where a MAH is submitting editorial changes as VNRA, under B.43 or C.9 scopes, the changes should be clearly identified in the UPD “submission comments” field as editorial changes, as well as any minor linguistic amendments introduced for each language. Alternatively, such listing may be provided as a separate annex to the be included VNeeS file. A declaration that the content of the concerned part of the dossier has not been changed by the editorial changes beyond the scope of the variation submitted should be also provided. Any changes not listed will not be considered as part of the variation application.
Changes that can be classified as a variation according to the Annex to the Implementing Regulation (EU) 2021/17 Variations Guidelines or to the EMA/CMDv Guidance on the details of the classification of variations requiring assessment are not considered editorial changes and should be submitted under the appropriate variation category.
In cases where any other ongoing procedures may affect the product information Annexes, the MAH is advised to contact the Agency in advance of submission or finalisation of the procedure(s) concerned.
In case of doubt, MAHs can contact the Agency via Service Now by selecting Veterinary Regulatory > Post-Authorisation-Vets in advance of the planned submission.
References
- Regulation (EU) 2019/6
- Commission Implementing Regulation (EU) 2021/17
- Guidance on the details of the classification of variations requiring assessment according to Article 62 of Regulation (EU) 2019/6 for veterinary medicinal products and on the documentation to be submitted pursuant to those variations
Variation scopes B.3.c, B.3.g, B.3.k, B.3.m, B.3.n, B.3.d and B.3.p of the Annex to the Commission Implementing Regulation (EU) 2021/17 deal with the deletion of a non-significant in-process control (IPC) test or specification parameter.
For the categories listed above and other variations related to specifications of active ingredients, excipients, finished product, packaging material or measuring or administration device, the deletion of an obsolete parameter is given as an example. For finished products, this is further exemplified by mentioning of odour and taste. Although it is not possible to give similar examples for all of the categories mentioned above, these examples serve as an indication of the types of changes considered to fall under these variation categories, regardless of whether the changes are related to in-process controls or specifications. The above-mentioned variation scopes or categories are therefore intended to be used for truly obsolete tests that are no longer part of normal specifications for newer products, but have remained for historical reasons in older products.
These variation categories are not intended to include changes in relation to revisions of the control strategy with an intention to minimise redundant testing of parameters and attributes (critical or non-critical) that are tested at different stages during the production, or cases where process/product characterisation performed after authorisation has shown that the attribute or parameter is non-critical. Such changes require regulatory assessment and are to be handled as VRA, as appropriate.
References
- Regulation (EU) 2019/6
- Commission Implementing Regulation (EU) 2021/17
- Guidance on the details of the classification of variations requiring assessment according to Article 62 of Regulation (EU) 2019/6 for veterinary medicinal products and on the documentation to be submitted pursuant to those variations