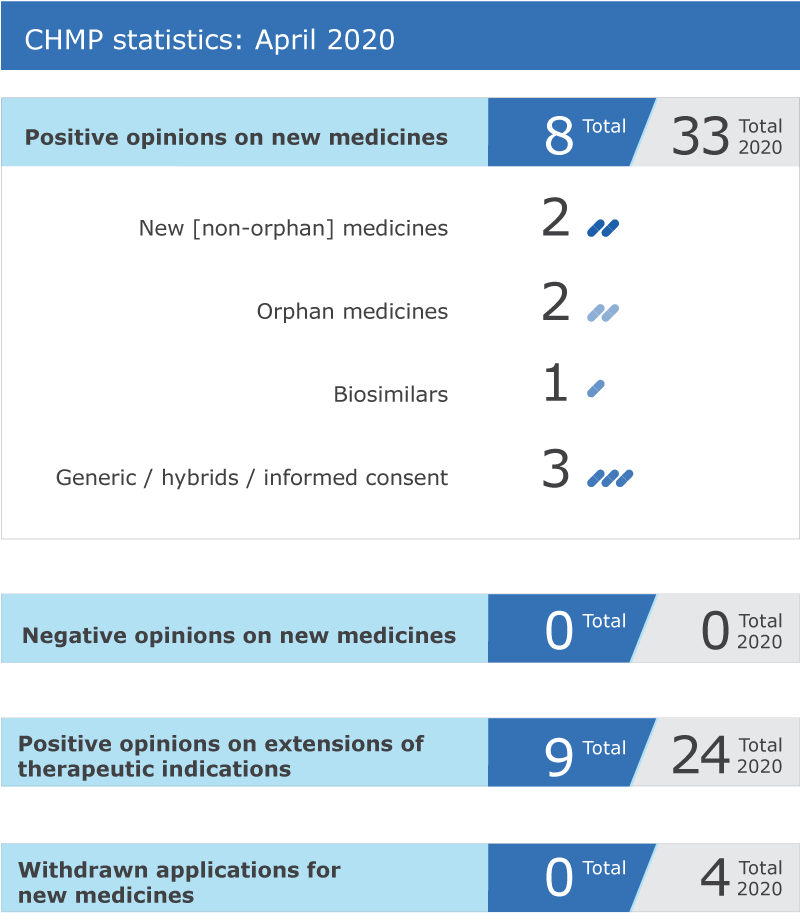
Eight new medicines recommended for approval
EMA’s human medicines committee (CHMP) recommended eight medicines for approval at its April 2020 meeting.
The Committee recommended granting a marketing authorisation for Enerzair breezhaler, the first triple combination therapy for the treatment of asthma which includes an optional smart electronic sensor. Enerzair breezhaler consists of a fixed dose combination of three active substances (indacaterol / glycopyrronium / mometasone furoate) in capsules, to be administered using an inhaler. An optional digital sensor collects data on a patient’s use and sends them to an app on a smart phone or another suitable device. For more information, see the press release in the grid below.
The CHMP also adopted a positive opinion for Zimbus Breezhaler (indacaterol / glycopyrronium / mometasone furoate) which is a duplicate of Enerzair Breezhaler for the treatment of asthma.
The Committee recommended granting a marketing authorisation for Daurismo* (glasdegib) for the treatment of acute myeloid leukaemia, a cancer of a type of immature white blood cell called myeloid cells.
Reblozyl* (luspatercept) received a positive opinion from the CHMP for the treatment of adults with transfusion-dependent anaemia associated with myelodysplastic syndromes (diseases in which the body produces large numbers of abnormal blood cells) or beta-thalassaemia (a blood disorder that reduces the production of haemoglobin).
The biosimilar medicine Insulin aspart Sanofi (insulin aspart) received a positive opinion for the treatment of diabetes mellitus.
The CHMP recommended granting a marketing authorisation for Cabazitaxel Accord (cabazitaxel), a hybrid medicine for the treatment of patients with hormone refractory metastatic prostate cancer previously treated with a docetaxel-containing regimen. Hybrid applications rely in part on the results of pre-clinical tests and clinical trials of an already authorised reference product and in part on new data.
Paliperidone Janssen-Cilag International (paliperidone), which was evaluated in an informed consent application, received a positive opinion for the treatment of schizophrenia. An informed consent application makes use of data from the dossier of a previously authorised medicine, with the marketing authorisation holder of that medicine giving consent for the use of their data in the application.
The generic medicine Fingolimod Accord (fingolimod) received a positive opinion for the treatment of relapsing-remitting multiple sclerosis with high disease activity.
Negative recommendation on new medicine following re-examination
The applicant for Hopveus (sodium oxybate) requested a re-examination of the Committee's negative opinion adopted at the October 2019 meeting. After considering the grounds for this request, the CHMP re-examined the initial opinion and confirmed its previous recommendation to refuse the granting of a marketing authorisation for this medicine, which was intended for the treatment of alcohol dependence.
For more information on this negative opinion, see the question-and-answer document in the grid below.
Nine recommendations on extensions of therapeutic indication
The Committee recommended extensions of indication for Braftovi, Cablivi, Carmustine Obvius, Ecalta, Harvoni, Kalydeco, Sovaldi, Taltz and Ultomiris.
The CHMP recommended the addition of a new pharmaceutical form (solution for injection) associated with a new strength and a new route of administration (subcutaneous injection into the abdomen) for Darzalex (daratumumab).
The Committee adopted a positive opinion recommending a new pharmaceutical form (sublingual film) associated with four new strengths for either sublingual or buccal use for Suboxone (buprenorphine / naloxone).
Outcome of review on ranitidine medicines
The CHMP recommended the suspension of all ranitidine medicines in the EU due to the presence of low levels of an impurity called N-nitrosodimethylamine (NDMA). Ranitidine medicines are used for reducing levels of stomach acid in patients with conditions such as heartburn and stomach ulcers. NDMA is classified as a probable human carcinogen (a substance that could cause cancer) based on animal studies. Available safety data do not show that ranitidine increases the risk of cancer, and any possible risk is likely to be very low. However, NDMA has been found in several ranitidine medicines above levels considered acceptable and there are unresolved questions about the source of the impurities.
For more information, see the public health recommendation in the grid below.
Outcome of review on fluorouracil medicines
The Committee recommended that patients should be tested for the lack of the enzyme dihydropyrimidine dehydrogenase (DPD) before starting cancer treatment with fluorouracil given by injection or infusion (drip) and related medicines containing capecitabine and tegafur. As treatment for severe fungal infections with flucytosine (another medicine related to fluorouracil) should not be delayed, testing patients for DPD deficiency before they start treatment is not required. Patients who completely lack DPD must not be given any fluorouracil medicines.
For more information, see the public health recommendation in the grid below.
Outcome of review on Picato
EMA has completed its review of Picato (ingenol mebutate), a gel for treating the skin condition actinic keratosis, and concluded that the medicine may increase the risk of skin cancer and that its risks outweigh its benefits. Picato is no longer authorised in the EU as the marketing authorisation was withdrawn on 11 February 2020 at the request of the company that marketed the medicine. For more information, see the public health recommendation in the grid below.
Agenda and minutes
The agenda of the April meeting is published on EMA's website. Minutes of the March 2020 CHMP meeting will be published in the coming weeks.
CHMP statistics
Key figures from the April 2020 CHMP meeting are represented in the graphic below.
* These products were designated as orphan medicines during their development. Orphan designations are reviewed by EMA's Committee for Orphan Medicinal Products (COMP) at the time of approval to determine whether the information available to date allows maintaining the medicines' orphan status and granting the medicines ten years of market exclusivity.
Positive recommendations on new medicines
| Name of medicine | Daurismo |
| International non-proprietary name (INN) | glasdegib |
| Marketing-authorisation applicant | Pfizer Europe MA EEIG |
| Therapeutic indication | Treatment of acute myeloid leukaemia |
| More information | Daurismo: Pending EC decision |
| Name of medicine | Enerzair Breezhaler |
| INN | indacaterol / glycopyrronium / mometasone furoate |
| Marketing-authorisation applicant | Novartis Europharm Limited |
| Therapeutic indication | Maintenance treatment of asthma in adults whose disease is not adequately controlled |
| More information | Enerzair Breezhaler: Pending EC decision
First triple combination therapy for asthma with optional electronic sensor |
| Name of medicine | Reblozyl |
| Common name | luspatercept |
| Marketing-authorisation applicant | Celgene Europe B.V. |
| Therapeutic indication | Treatment of adults with transfusion-dependent anaemia associated with myelodysplastic syndromes or beta-thalassaemia |
| More information | Reblozyl: Pending EC decision |
| Name of medicine | Zimbus Breezhaler |
| INN | indacaterol / glycopyrronium / mometasone furoate |
| Marketing-authorisation applicant | Novartis Europharm Limited |
| Therapeutic indication | Maintenance treatment of asthma in adults whose disease is not adequately controlled |
| More information | Zimbus Breezhaler: Pending EC decision |
Positive recommendation new generic medicine
| Name of medicine | Fingolimod Accord |
| INN | fingolimod |
| Marketing-authorisation applicant | Accord Healthcare S.L.U. |
| Therapeutic indication | Treatment of relapsing-remitting multiple sclerosis with high disease activity |
| More information | Fingolimod Accord: Pending EC decision |
Positive recommendation on new biosimilar medicine
| Name of medicine | Insulin aspart Sanofi |
| INN | insulin aspart |
| Marketing-authorisation applicant | sanofi-aventis groupe |
| Therapeutic indication | Treatment of diabetes mellitus |
| More information | Insulin aspart Sanofi?????: Pending EC decision |
Positive recommendation on new hybrid medicine
| Name of medicine | Cabazitaxel Accord |
| INN | cabazitaxel |
| Marketing-authorisation applicant | Accord Healthcare S.L.U. |
| Therapeutic indication | treatment of patients with hormone refractory metastatic prostate cancer previously treated with a docetaxel-containing regimen |
| More information | Cabazitaxel Accord?????: Pending EC decision |
Positive recommendation on new informed consent
| Name of medicine | Paliperidone Janssen-Cilag International |
| INN | paliperidone |
| Marketing-authorisation applicant | Janssen-Cilag International N.V. |
| Therapeutic indication | Treatment of schizophrenia |
| More information | Paliperidone Janssen-Cilag International?????: Pending EC decision |
Negative recommendation on new medicine following re-examination
| Name of medicine | Hopveus |
| INN | sodium oxybate |
| Marketing-authorisation applicant | D&A Pharma |
| Therapeutic indication | Treatment of alcohol dependence |
| More information | Hopveus?????: Pending EC decision |
Positive recommendations on extensions of indications
| Name of medicine | Braftovi |
| INN | encorafenib |
| Marketing-authorisation holder | Pierre Fabre Medicament |
| More information | Braftovi: Pending EC decision |
| Name of medicine | Cablivi |
| INN | caplacizumab |
| Marketing-authorisation holder | Ablynx N.V. |
| More information | Cablivi: Pending EC decision |
| Name of medicine | Carmustine Obvius |
| INN | carmustine |
| Marketing-authorisation holder | Obvius Investment B.V. |
| More information | Carmustine Obvius: Pending EC decision |
| Name of medicine | Ecalta |
| INN | anidulafungin |
| Marketing-authorisation holder | Pfizer Europe MA EEIG |
| More information | Ecalta: Pending EC decision |
| Name of medicine | Harvoni |
| INN | ledipasvir / sofosbuvir |
| Marketing-authorisation holder | Gilead Sciences Ireland UC |
| More information | Harvoni: Pending EC decision |
| Name of medicine | Kalydeco |
| INN | Ivacaftor |
| Marketing-authorisation holder | Vertex Pharmaceuticals (Ireland) Limited |
| More information | Kalydeco: Pending EC decision |
| Name of medicine | Sovaldi |
| INN | sofosbuvir |
| Marketing-authorisation holder | Gilead Sciences Ireland UC |
| More information | Sovaldi: Pending EC decision |
| Name of medicine | Taltz |
| INN | ixekizumab |
| Marketing-authorisation holder | Eli Lilly Nederland B.V. |
| More information | Taltz: Pending EC decision |
| Name of medicine | Ultomiris |
| INN | ravulizumab |
| Marketing-authorisation holder | Alexion Europe SAS |
| More information | Ultomiris: Pending EC decision |
Important recommendations on new strengths, formulations or routes of administration
| Name of medicine | Darzalex |
| INN | daratumumab |
| Marketing-authorisation holder | Janssen-Cilag International N.V. |
| More information | Darzalex?: Pending EC decision |
| Name of medicine | Suboxone |
| INN | buprenorphine / naloxone |
| Marketing-authorisation holder | Indivior Europe Limited |
| More information | Suboxone: Pending EC decision |
Public-health recommendations
| Name of medicine | Fluorouracil and fluorouracil related substances (capecitabine, tegafur and flucytosine) containing medicinal products |
| INN | capecitabine, fluorouracil, tegafur, flucytosine |
| More information | EMA recommendations on DPD testing prior to treatment with fluorouracil, capecitabine, tegafur and flucytosine |
| Name of medicine | Picato |
| INN | ingenol mebutate |
| More information | Risks of Picato for actinic keratosis outweigh benefits |
| Name of medicine | Ranitidine-containing medicinal products |
| INN | ranitidine |
| More information | Suspension of ranitidine medicines in the EU |
Outcome of arbitration procedure
| Name of medicine | Carbamazepin Tillomed |
| More information | EMA recommends authorisation of Carbamazepin Tillomed (carbamazepine, 200 and 400 mg prolonged release tablets) in the EU |
Start of re-examination of arbitration procedure
| Name of medicine | Budesonide SUN |
| INN | budesonide |
| More information | EMA recommends refusal of authorisation for Budesonide Sun (budesonide, nebuliser suspension) in the EU |
Other opinion
| Name of medicine | Keytruda |
| INN | pembrolizumab |
| Marketing-authorisation applicant | Merck Sharp & Dohme B.V. |
| More information | Questions and answers on the use of Keytruda alone in non-small cell lung cancer with low levels of PD-L1 |